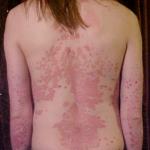
"Clinical trial" is just a nice-sounding term for "human experiment." We don't like to use that term because it carries a lot of baggage, like the torturous and murderous "experiments" conducted by Nazi doctor Josef Mengele.
clinical trials
Not in our wildest imagination would anyone have thought that we would have two 90+% effective COVID vaccines by mid-November. Most vaccine experts were estimating 50%, maybe 70% if everything went right. Wow!
Several months ago, I wrote an article about how UK health authorities have more guts than their U.S. counterparts.
Another day, another coronavirus controversy. Well, maybe several controversies. The latest stories keep coming so fast, that it's difficult to know what's accurate and what isn't.
One of the most famous passages from the Bible is Ecclesiastes 3:1-8, which inspired the Byrds' song Turn Turn Turn. Here is an excerpt:
Today, Vladimir Putin announced that Russian regulators have approved the world's first coronavirus vaccine. He's so confident in it that he claims that one of his daughters has been vaccinated already.
There are a plethora of drugs and vaccines in the pipeline to treat or prevent COVID-19, the disease caused by the novel coronavirus, SARS-CoV-2. How many of them are likely to be successful?
The endless daily reports of possible vaccines and potential drugs to treat coronavirus all mention the need for clinical trials before the drug or a vaccine can be approved for use, but without making it clear what the trial involve.
# From The Latest Hydroxychloroquine Data, As of April 11.
When you practice medicine, you are often tethered to your smartphone.