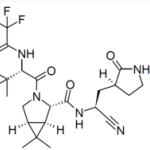
Today, NBC News managed to spoil the coming-out party of a desperately needed, lifesaving drug with its lead headline of the morning:
"Pfizer antiviral pills may be risky with other medications."
direct acting antiviral drugs
The "COVID era" is reteaching us a lesson that was learned in the 1990s and 2010s – that an effective direct-acting antiviral (DAA) medication can work wonders in controlling or eliminating a serious viral infection.
Putting aside the usual anti-pharma narrative for a moment, Merck, which is seeking emergency use authorizat
As Professor Katherine Seley-Radtke (1) and I wrote last July in an op
Remember back in the early days of COVID when we knew little about the disease and had nothing to treat it with? Remdesivir (brand name Veklury®) seemed promising for a while, but as I wrote last year:
The race to discover anti-COVID drugs, just like the race to discover any drugs, has been long (1) and frustrating.