The "COVID era" is reteaching us a lesson that was learned in the 1990s and 2010s – that an effective direct-acting antiviral (DAA) medication can work wonders in controlling or eliminating a serious viral infection. Much like the anti-HIV drugs completely changed the course of the terrifying AIDS epidemic and the anti-HCV drugs provided a reliable cure for hepatitis C infection, anti-COVID drugs keep getting better and better, perhaps to the point where COVID becomes little more than a masked memory.
Last month I argued that Merck's molunipravir should be granted emergency use authorization (EUA) because the drug, which inhibits viral RNA synthesis, knocked down serious COVID by 50% when given early without short-term adverse effects, probably booting Gilead's remdesivir – which must given by IV infusion – into pharmaceutical oblivion.
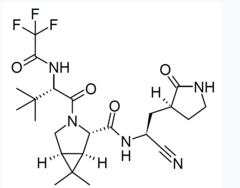
Today, Pfizer upped the ante when the company announced that its Main Protease (Mpro) aka the 3CL protease inhibitor (3) Paxlovid reduced severe COVID (hospitalization or death) by 89% (!). As was the case with the first announcement of the efficacy of the Pfizer mRNA vaccine this took everyone by surprise, even Pfizer's chairmain Albert Bourla.
“I think this medicine will change the way things are happening right now that will save millions and millions of lives, it has the potential to do it...The very high efficacy comes even to us as a surprise, exceeds our most visionary expectations we had for that."
Albert Bourla in a CNBC interview
Here's a summary of the clinical trial results:
- The study (randomized, double-blinded) included adults with at least one underlying medical condition and confirmed COVID.
- Paxlovid, formerly PF-07321332, was given along with ritonavir (1) to patients within three days of infection (2).
- In the drug-treated group (389 people) 3 people required hospitalization, none died.
- In the control group (385 people) 27 people required hospitalization, 7 died.
- Adverse events were similar in the treated and placebo groups.
- These are interim results; the trial was stopped early
I hate to gloat (OK, maybe "hate" is too strong a word) but Professor Katherine Seley-Radtke and I called this well more than a year ago in an opinion piece titled Vaccines might not be the only way to treat COVID-19, which appeared in the July 1st, 2020 edition of the Baltimore Sun.
Egos aside, these results, barring unforeseen events, could very well stop this hideous pandemic in its tracks. Or, at the very least, make COVID into something like a bad cold or mild flu. We've needed something like this for a long time. We should all hope that these results hold up in larger trials. I expect they will.
"Hopefully, this is the first of many therapeutics to come that will help us finally get back to normal, but also allow us to be proactive, rather than reactive, in fighting other future pandemics"
Prof. Kathie Seley-Radtke, Ph.D.
Professor, Chemistry & Biochemistry University of Maryland,
NOTES:
(1) When the drug was given within five days, the numbers were similar.
(2) Ritonavir comes from the early days of AIDS. It was originally developed an a HIV protease inhibitor but was later found (in low dose) to extend the half-life of other protease inhibitors by inhibiting the liver enzymes that were "chewing" up the drug.
(3) Remdesivir and molunipravir both act by inhibiting viral RNA synthesis. Paxlovid acts by inhibiting the processing (cutting) of the large polyprotein that is produced by ribosomes into smaller functional proteins, responsible for the structure and enzymatic activity of new virus particles.



