Some Good News
COVID-19, at least in the US, is steadily being reigned in. Even the nasty contagious Delta variant, aka B.1.617.2, which originated in India and is now strutting its stuff here is well-covered by the current vaccines. But if you're sitting on the fence about getting vaccinated it's probably time to hope off:
“It’s more transmissible than the Alpha variant, or the U.K. variant, that we have here...we saw that quickly become the dominant strain in a period of one or two months, and I anticipate that is going to be what happens with the Delta strain here.”
CDC Director Dr. Rochelle Walensky, “Good Morning America.” June 18, 2021
We also dodged the flu season this year.
Could there possibly be a worse time to be writing about another respiratory virus? Enough already..
There was no flu season this year.
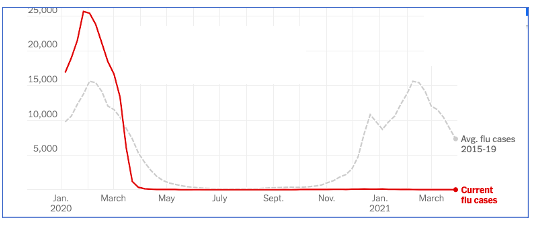
Original Graphic: New York Times. Data from CDC
Most of us managed to dodge norovirus.

Norovirus outbreaks. Source: CDC. The gray hatch line represents 2019-2020, the red solid line is 2020-2021. The solid gray area represents the range from 2012-2019.
Not such good news - An old enemy makes a comeback
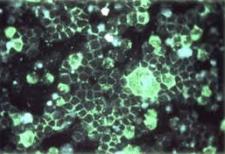
While RSV, short for respiratory syncytial virus, doesn't come up in routine conversation, it's a pretty nasty virus that. Although the name may be obscure the virus is not. The 2019 RSV burden – 14,000 deaths and 177,000 hospitalizations – is roughly one-third to one-half that of influenza in a given year.

Respiratory Syncytial Virions (blue) Source: Wikimedia Commons
It s not an infection that sits on the tip of your tongue, but an effective treatment for respiratory syncytial virus (RSV) is an unmet medical need that has been shrugging off attempts to control it for many years.
Although the infection can manifest itself as nothing but a bad cold, rhinovirus, the pathogen that causes colds, is unrelated to RSV. And, unlike a cold, RSV can be fatal, especially in young children and older adults. There is no specific treatment or prevention for RSV infection, but it looks like this may change, thanks to a very promising vaccine that is made up of virus-like particles (VLPs).
VLPs may sound familiar, since they are the rage in new vaccine technology. They are also being used in the development of vaccines against norovirus (the stomach flu,) and show great promise in finally attaining the long sought-after universal flu vaccine something that we discussed recently:
VLPs are artificial constructs that contain only the shell of the virus, which produces the same (or similar) antigens that are present in the actual virus. But, unlike many other vaccines, VLPs do not contain any virus and therefore cannot be infectious (this is very rare even with traditional vaccines). Nor do they even require a sample of the virus. They are typically made in mammalian or plant cells using the DNA sequence of the actual virus.
Virtually all children have had an RSV infection at some point in their lives. In most cases it is similar to a bad cold, but not always. According to the CDC, the virus is responsible for:
· 57,527 hospitalizations among children younger than 5 years old
· 2.1 million outpatient visits among children younger than 5 years old
· 177,000 hospitalizations and 14,000 deaths among adults older than 65 years
To put this in perspective, the morbidity and mortality of RSV is virtually identical to that of influenza. This is far from trivial.
But, it looks like help may be on the way: Novavax, a Maryland-based biopharmaceutical company is leading the pack. Their Phase II vaccine has been shown to prevent 46 percent of symptomatic RSV infections in older adults, and a 64 percent reduction in severe infection.
Andrew Pavia, speaking for Infectious Diseases Society of America, said, "It could be a major breakthrough [but] It's not time to break out the champagne."
About one-third of new drugs that make it through Phase II trials go down in flames during Phase III. They shouldn't be picking out the color of their new Lexus quite yet.



