Having spent a decade (of pure futility) in hepatitis C (HCV) research in my former career, I have spent a fair amount of time since then writing about the astounding progress made over a 25-year period. Very effective antiviral drugs, Sovaldi being the first, stopped the virus its tracks. Most of the time.
But not all of the time. One reason for this is that there are a number of strains of the virus, which are called genotypes, and some of them are harder to treat than others. The predominant genotype is highly dependent upon geography.
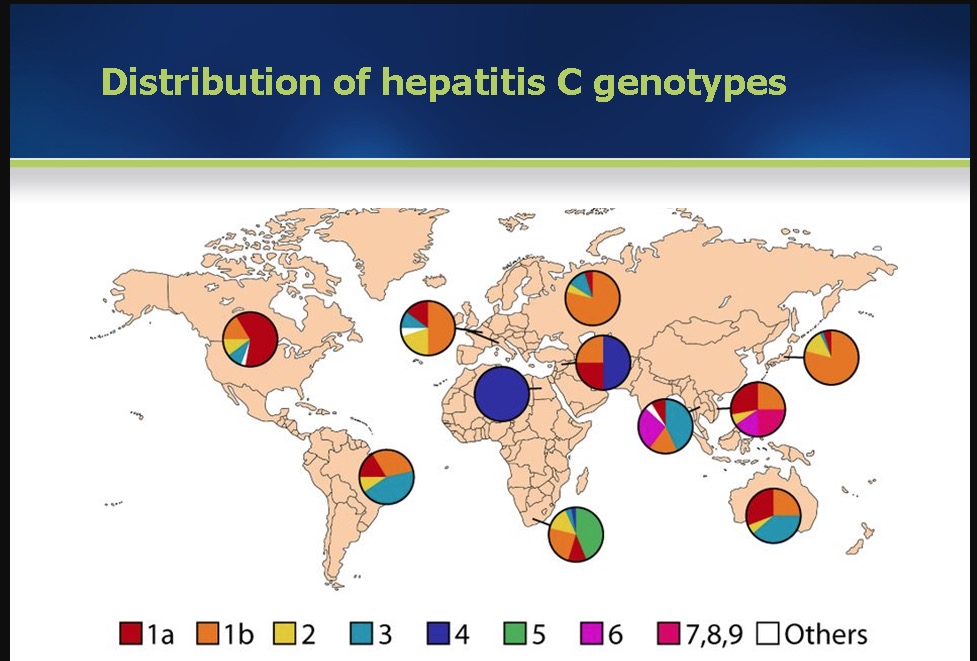
Figure 1: The worldwide distribution of hepatitis C genotypes. Source: Johns Hopkins
Given that genotype 1 causes the most HCV infections in the US (about 75%) and also worldwide (46%), most initial research was geared toward stopping it. But genotype 3, which is the second most prevalent strain worldwide, causes about 10 percent of the cases in the US and 22% in the world. It also causes more severe liver disease and is more difficult to treat. Patients with genotype three infections are more likely to develop liver cancer.
As is the case with the AIDS cocktails, combinations of direct-acting antiviral drugs that work by attacking different stages in viral replication, also worked best against HCV. One such cocktail called Vosevi was just approved by the FDA after an accelerated review for the treatment of patients who are infected with genotypes 1-6. In two trials the drug enabled patients to attain a sustained virological response (SVR) (1) with a success rate of 96-97 percent. Some of these patients had already failed Sovaldi therapy. Pretty amazing.
Vosevi contains:
- Sofosbuvir (Solvaldi) - inhibits RNA replication. (Molecular target is called NS5B) (2)
- Velpatasvir - inhibits the formation of a replication complex, which brings together multiple components that are necessary for making new viruses. (Molecular target is called NS5A)
- Voxilaprevir - an inhibitor of HCV protease—the enzyme that cuts up the single large protein that is originally produced in the host cell into smaller essential viral proteins. (Molecular target is called NS3A/4)
We have come a long way. HCV was first identified in 1989. Sovaldi—the first really good drug to stop the virus—was approved in 2013. Yes, 24 years of research by dozens of companies, which yielded one drug (3). Sovaldi was a quantum leap forward. Vosevi, which some utterly clueless critics of the pharmaceutical industry (4) dismiss as a "me-too" drug is a significant improvement. It mops up whatever the older drugs can not.
It took 24 years to find the first drug that slammed the brakes on a very significant infection and 28 years to bring it to a near-stop. This is drug discovery at its best. Unless you are (or were) part of the industry it is difficult to appreciate
Notes:
(1) A sustained virological response is defined as the patient being virus-free 12 weeks following cessation of treatment. This is considered to be a cure.
(2) NS means non-structural—the viral proteins that are required for replication.
(3) Two direct acting antivirals from Schering and Vertex were approved in 2011. They both disappeared quickly after Solvaldi became available.
(4) Yes, Jeffrey Sachs, Marcia Angell, and Sid Wolfe. I'm talking about you.




