There is only one shingles prevention vaccine currently on the market. And while it is effective, it does have a glaring drawback: over time it becomes less so – even to the point of uselessness – beginning less than a decade after vaccination.
However, there appears to be a better alternative on the horizon, with Wednesday's advisory panel approval from the Food and Drug Administration of a new vaccine developed by GlaxoSmithKline.
Panel members, saying they were "very impressed" with the drug for adults 50 and over, called Shingrix, voted 11-0 in favor of approval. While the FDA is not bound by the advisory group's recommendation, in instances like this where there is no objection chances for the administration's full approval appear to be exceedingly good. A vote is expected to take place in October.
The panel, formally known as the Vaccines and Related Biological Products Advisory Committee, agreed with GSK's contention that its vaccine was an improvement over Zostavax, Merck's vaccine that was approved in 2006. While the efficacy of Zostavax falters over time, GSK said, Shingrix (also known as HZ/su) has greater staying power and it can protect a wider range of older adults, as the table indicates.
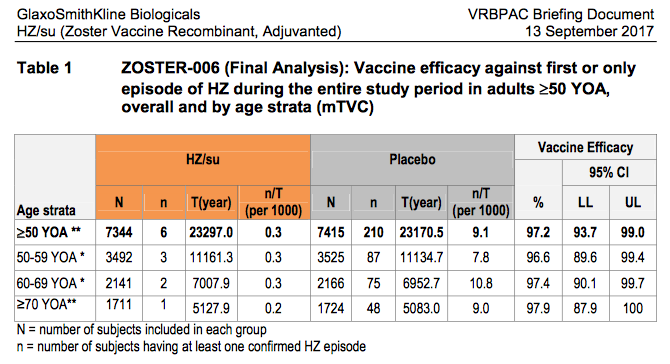
In GSK's briefing document for the committee, referring to shingles – clinically known as Herpes Zoster, or HZ – the company wrote that while "Zostavax offers the opportunity for prevention of HZ, its efficacy is not consistent across all age ranges, and effectiveness wanes to non-significant levels 8 to 11 years after vaccination."
Following its presentation, during which drug company officials said Shingrix could not only remain effective longer than Zostavax, but also that it reduces nerve pain when taken after an HZ outbreak, the committee was reportedly won over. It also has shown to be effective for those over 70 years old. However, the committee was concerned that a large percentage of trial subjects were white, while expressing the desire that more testing be done involving a wider range of patient subgroups.
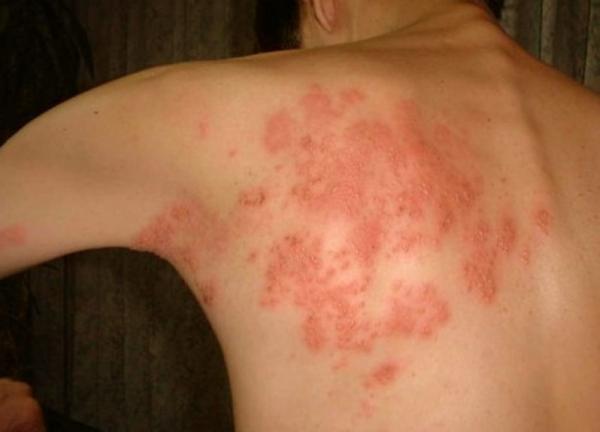
Shingles, which affects an estimated one million Americans each year, is most often seen in middle-aged adults and those older whose immune system is compromised for various reasons. It appears as a blistering rash and can deliver acute nerve pain that can linger for months, and up to several years.
Roughly 1 in 3 older adults in their lifetime will experience shingles, which is a result of a recurrence of the varicella-zoster virus, which causes chickenpox.
GSK is also actively seeking approval for Shingrix in Australia, Japan, Canada and in the European Union.