Remember the great news last fall when the FDA unanimously approved a new anti-shingles vaccine, which was shown to be much more effective than the available alternative? Well, if you do, it appears that you're not alone. Not by a long shot.
Because with so many middle-aged and elderly folks having already gone out to receive the first of a two-part vaccination, there's simply not enough of the medicine, named Shingrix, to go around. So instead of getting their second shot to complete the series, what many have received instead is a spot on a waiting list.
“It is not a manufacturing issue,” says spokesman Sean Clements, representing the drug's manufacturer GlaxoSmithKline “The demand for this vaccine has been unprecedented.”
The reason for that demand is in the vaccine's value: Shingrix has been shown to be more than 90 percent effective, and much longer lasting than its best competitor, Zostavax, which works about 50 percent of the time and often begins to wear off less than a decade after being administered.
Since the manufacturer states that "99% of people over 50 years of age are living with the virus that causes shingles" the Centers for Disease Control recommends that those in this demographic get both shots within six months. It has posted a list of guidelines, which address frequently-asked questions, including how to proceed if you are unable to receive the second dose within the six-month window. (It's OK, just don't delay. You don't have to receive the first shot again.)
 In case you're wondering whether a single dose is effective on its own, even the manufacturer isn't aware if it works that way because Shingrix was created as a two-step course and never tested as a single-shot vaccine.
In case you're wondering whether a single dose is effective on its own, even the manufacturer isn't aware if it works that way because Shingrix was created as a two-step course and never tested as a single-shot vaccine.
Earlier this month, two U.S. Senators urged GSK to significantly scale up supply of Shingrix. This week, the company says it is doing just that.
“We are shipping large volumes of Shingrix every two to three weeks and expect this schedule to continue for the remainder of the year,” added GSK's Clements, according to the Washington Post. "Going forward, providers and patients can feel confident that more doses are being made available regularly and that they will be able to find the vaccine to complete their two-dose series.”
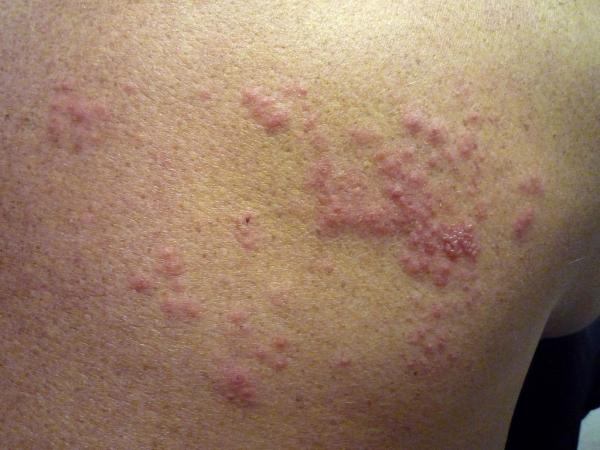
Shingles, which affects an estimated one million Americans each year, is most often seen in middle-aged adults and those older whose immune system is compromised for various reasons. It appears as a blistering rash and can deliver acute nerve pain that can linger for months, and up to several years.
Roughly 1 in 3 older adults in their lifetime will experience shingles, which is a result of a recurrence of the varicella-zoster virus, which causes chickenpox. The CDC recommends those over 50 get the Shingrix vaccine even if they were previously stricken with shingles, or had received Zostavax, or can't recall if they ever had chickenpox as a child or younger adult.