There's ozone, and then there's ozone.
Is ozone good for us? Yes.
Is ozone bad for us? Yes.
All of which means that the next time the conversation turns to ozone, it's good to know the context before you weigh in.
But I know what most of you are thinking: "Look pal, who's got time to talk about ozone? And, more importantly, it doesn't come up. So why should I even pay attention to the details?" Well, maybe because it's increasingly being used to combat the spread of infection, including methicillin-resistant Staphylococcus aureus, more commonly known as MRSA.
Prior to that, when we thought of ozone it was frequently in reference to the layer in the upper atmosphere that protects us from the sun's harmful ultraviolet, or UV, rays. As we know it's become an ubiquitously important topic, as humankind struggles with managing the "hole" in the ozone layer created by vapors emanating from manmade chemicals. So as the Environmental Protection Agency terms it, this "stratospheric ozone" is "good ozone" that "occurs naturally."
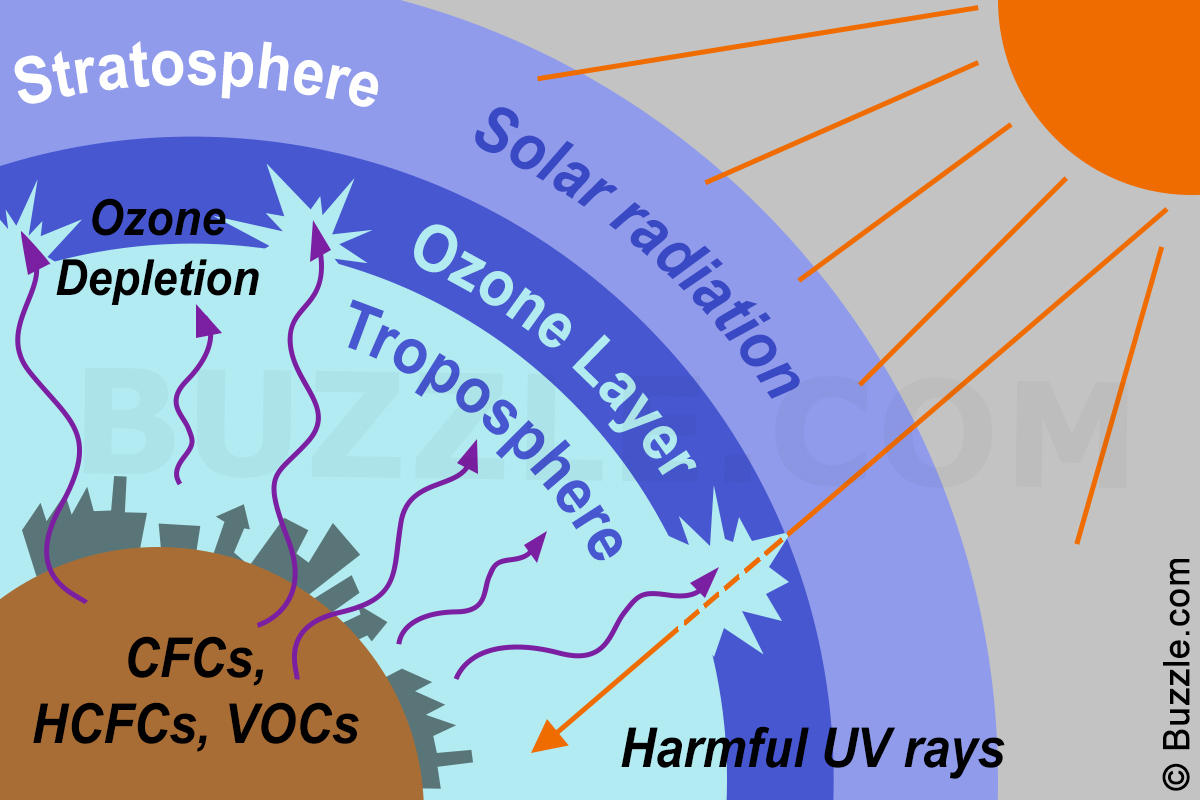 But hold that thought because we also produce "bad ozone," which is "created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOC)," the EPA tells us. "This happens when pollutants emitted by cars, power plants, industrial boilers, refineries, chemical plants, and other sources chemically react in the presence of sunlight. Ozone at ground level is a harmful air pollutant," in the troposphere, and "it is the main ingredient in 'smog.'"
But hold that thought because we also produce "bad ozone," which is "created by chemical reactions between oxides of nitrogen (NOx) and volatile organic compounds (VOC)," the EPA tells us. "This happens when pollutants emitted by cars, power plants, industrial boilers, refineries, chemical plants, and other sources chemically react in the presence of sunlight. Ozone at ground level is a harmful air pollutant," in the troposphere, and "it is the main ingredient in 'smog.'"
Now to that atmospheric dichotomy we add the type of ozone that cleanses and disinfects – the basis for a stepped-up approach to battling the insidious spread of MRSA.
With sports locker rooms having witnessed the spread of the infection – which resists antibiotics, has debilitating health effects and can even prove fatal – organizations are turning to ozone-generating sanitizing machines that clean and deodorize all sorts of gear, including sports equipment. As the New York Times reported recently, Colgate University, in its determination to prevent the spread of MRSA and other bacteria, has begun using these $14,000 units.
 Sports-O-Zone (pictured here), which is made by an Indiana-based company, is billed as a machine that produces "ozone onsite so there is no need for consumables. Since ozone is a gas, it can deodorize and sanitize those hard to reach places on your equipment, making sure you get a greater than 99.9% kill rate of bacteria, viruses and molds."
Sports-O-Zone (pictured here), which is made by an Indiana-based company, is billed as a machine that produces "ozone onsite so there is no need for consumables. Since ozone is a gas, it can deodorize and sanitize those hard to reach places on your equipment, making sure you get a greater than 99.9% kill rate of bacteria, viruses and molds."
OK, good to know. Now, next up, chemistry. In this context, what is ozone? What's its composition?
"Ozone is produced when O2 is energized and split into two monatomic (O1) molecules," explains the Centers for Disease Control. "The monatomic oxygen molecules then collide with O2 molecules to form ozone, which is O3. Thus, ozone consists of O2 with a loosely bonded third oxygen atom that is readily available to attach to, and oxidize, other molecules. This additional oxygen atom makes ozone a powerful oxidant that destroys microorganisms."
These ozone machines have been added to public high schools and NFL equipment rooms alike. And as the Times reported, after a Bowdoin College student-athlete contracted a case of MRSA a decade ago, the Maine-based school hasn't had another incident since it started using the ozone unit.
MRSA, once confined to hospitals and healthcare facilities, has infiltrated college and university locker rooms nationwide. And it's being taken very seriously.
"With the advent of MRSA and related bacterial, viral and fungal skin infections reported in recent years, it is critical to keep these surfaces routinely cleaned and checked for germs," states the National Athletic Trainers' Association, in a message posted at the beginning of this academic year. "Athletes must be discouraged from sharing towels, athletic gear, water bottles, disposable razors and hair clippers. All clothing and equipment should be laundered and/or disinfected on a daily basis."
So when breathing in ozone – which can cause wheezing by constricting muscles in the airways – it can be your enemy. But as a disinfectant, it can be your friend. It's just good to know which one you're dealing with.




