Quick quiz: What is the world's most deadly human pathogen for which there is no vaccine? Since the title, unfortunately, must precede the article, you already know the answer. Norovirus, which is incorrectly called the "stomach flu," isn't a virus that most people would associate with death (although you may prefer to be dead if you catch it), but its annual death toll is estimated to be 200,000, mostly due to dehydration in developing areas that lack clean water.
But a group at the Washington University School of Medicine in St. Louis may have discovered what makes the bug so nasty which may also represent a possible way to exploit this nastiness to help discover a badly-needed drug or vaccine.
To add to its splendid array of qualities, norovirus, which got its name from Norwalk, Ohio, where the virus was first isolated after a 1968 outbreak, is the most contagious virus of all. It only takes about ten virus particles to infect you (this is a crazy low number), and it can be shed to infect others long after the symptoms, nausea, vomiting, diarrhea, and head spinning around, are gone. As if this little monster isn't bad enough, it can be spread through the air, contaminated surfaces (it's also hard to kill) and food handlers (fecal-oral). Norovirus is the number one cause of food poisoning in the US.
So, it's a pretty safe bet that an overwhelming majority people would choose "NO" over "YES" if given the choice of whether they would like to catch the damn thing (1).
The Washington University researchers just published a paper in Science which explains why the virus is both so contagious and also why it sticks around so long. It loves a particular type of cell in your gut. According to Dr. Craig B. Wilen and colleagues, a rare cell called a tuft cell, found in the small intestine, provides the virus with a place to replicate as well as serving as a reservoir that causes some people to remain contagious weeks after they feel better. And since the gut microbiome plays a role in regulating tuft cells, this a rare case of antibiotics being able to impact a viral disease. At least in mice.
Adding to its treachery, norovirus, although it replicates like a madman in your gut, can't be grown in cells in a dish, which makes finding a drug to stop it very much more difficult to find (2). So the group used murine norovirus (MNoV), the mouse version of the human bug, and grew it in mice instead. As expected, the MNoV infected the mice, but only the tuft cells.
"In a single mouse, for example, maybe 100 cells will be infected, which is very few compared with other viruses such as the flu."
Craig Wolen, MD, PhD
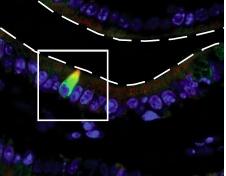
Figures 1 is a representation of the intestinal epithelium. A number of different cells are embedded in the epithelium. Figure 2 is an actual image of mouse epithelial cells.

Figure 1. (Right) A schematic drawing of a section of the intestinal epithelium. (Center) Magnified image. The red arrows indicate tuft cells embedded in the epithelium. (Right) Cells that are embedded in the epithelium. The tan area is called the lamina propria, which is on the other side (the outside) of the epithelium contains various immune cells. Adapted from: Nature Mucosal Immunology volume 9, pages 1353–1359 (2016) doi:10.1038/mi.2016.68
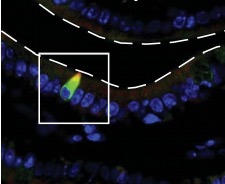
Figure 2. A single tuft cell in the epithelium. The color comes from a fluorescent protein that specifically binds to these cells. Source: Nature
The role of the tuft cell is the essence of this discovery. These cells are a component of our gut immune system; they proliferate in the presence of intestinal parasites to fight off parasitic infections. In doing so, the proliferation triggers an immune response, which probably makes the symptoms of norovirus worse. The authors speculate that treatment of the mice with broad-spectrum antibiotics would kill multiple pathogens in the gut and alter the biome so that the proliferation of the tuft cells would be prevented. This is an unusual situation where antibiotics can have an impact, albeit an indirect one, on a viral infection.
But don't run out and stock up on antibiotics. Killing everything in your intestinal biome is a very poor idea. But the idea of blocking the infection of a very small subset of intestinal cells to prevent or minimize norovirus infection is intriguing. Let's hope this strategy points us to a vaccine or therapy for this very unpleasant viral infection.
NOTES:
(1) But they don't get that choice. 20 percent of the US will get it each year, making it the second most common infection after the cold. And getting it gives you little immunity. You can get the damn thing again the same year.
(2) Some viruses grow just fine in isolated cells, and some do not. The reason for this is unclear. For example, the inability to grow hepatitis C virus outside of a liver hampered research in this area.