I have previously written about the concept of "drug-drug interactions." I put the term in quotes because it is not strictly accurate. The name implies that two drugs are somehow combining inside your body and forming something else that could be harmful (or helpful). No, this is not how it works. A more accurate term would be "Up- or down-regulation of hepatic metabolizing enzymes that are encoded by the cytochrome P450 (CYP)1A2, 2C9, 2D6, 3A4, and 3A5 genes in response to an external stimulus." But that doesn't exactly roll off the tongue.
So, let's keep it simple. The liver is by far the most important organ we have for metabolizing (breaking down) drugs, chemicals, proteins, etc. that we ingest (1). Some drugs stimulate (upregulate) the liver to produce more cytochrome P450 enzymes (CYPs) resulting in a faster metabolism and excretion of other drugs. This can result in an inadequate response at a dose that would normally work just fine.
On the other hand, some drugs tie up (inhibit) the CYP enzymes making them less effective in breaking down other drugs. This can result in higher concentrations of other drugs and a longer half-life then was intended. When certain CYPs are inhibited by other drugs this can result in concentrations in the blood that are significantly higher than normal, which can result in overdoses. More on this below.
NOMENCLATURE
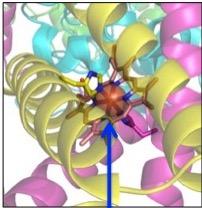
The numbers and letters used while discussing metabolism can seem a bit daunting. But it's really not so bad. CYP is short for cytochrome P450 (2). Cytochromes are proteins that contain heme - a complex between iron and molecule that holds it in place. (Figure 1)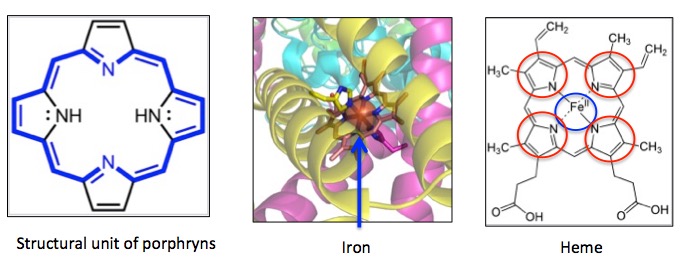
Figure 1. Two different depictions of heme. (Left) A porphyrin molecule. Porphyrins are large rings containing four smaller rings (Right, red circles), each having a nitrogen pointing toward to middle of the molecule. This makes them perfectly designed to bind to iron. (Center) Three-dimensional ribbon diagram showing iron (orange globe) being held in place by a porphyrin molecule. (Right) Two dimension representation of heme - a porphyrin molecule bound to iron (blue circle). Note the perfect fit of iron inside the large ring. The red circles are the four subunits that are bound together to make the porphyrin. Images: Wikipedia.
Now that that's out of the way, let's look at what happens in real life when the presence of one drug can have profound effects on a second drug. I am using opioids as an example because they are newsworthy and also because their interactions with other drugs have been extensively studied.
DRUGS THAT AFFECT BLOOD LEVELS OF OPIOIDS
1. Voriconazole and Oxycodone
Voriconazole is an oral antifungal medication, which is a potent inhibitor of CYP3A4 - the most important member of the CYP family responsible for metabolizing oxycodone. A 2009 study (3) showed that when Voriconazole was given to healthy patients along with oxycodone the blood levels of the oxycodone were four-times higher than normal. So, co-administration of these two drugs will increase the amount of circulating oxycodone and it will also stay in the blood longer. It is not difficult to see why one must pay careful attention when people are taking opioids along with CYP3A4 inhibitors. The chances of an overdose automatically become higher.
2. Rifampin and Oxycodone
Conversely, the tuberculosis medication rifampin is known to be an inducer of CYP3A4. In other words, it "signals" the liver that it needs to make more of CYP3A4. So, it would be expected that co-administration of oxycodone and rifampin would result in lower (possibly inadequate) blood levels of oxycodone. Indeed, a 2009 study (4) showed that co-administration of rifampin with oxycodone resulted in far lower (and possibly inadequate) levels of oxycodone.
METABOLISM CHANGES EVERYTHING
3. Oxycodone vs. Itself
This one's a bit complicated. Although you may think that Percocet (oxycodone plus Tylenol) is just a more powerful analgesic than Vicodin (hydrocodone plus Tylenol) try telling this to your liver (Figure 2).
The metabolic "story" of oxycodone is simple. The CYP3A4 enzyme chews off the methyl group on nitrogen (red circle), converting it to noroxycodone. But noroxycodone is not an active analgesic, so as the oxycodone gets metabolized it's ability to control pain decreases (Figure 2).
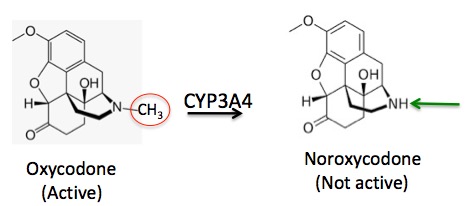
Figure 2. The metabolism of oxycodone to noroxycodone. The methyl group (red circle) is removed and replaced by a hydrogen (green arrow).
4. Hydrocodone vs. Itself
But in the case of hydrocodone something very different happens. Hydrocodone is the favorite snack of CYP2D6, which goes after a different methyl group - the one attached to oxygen (red circle), replacing it with a hydrogen atom (green arrow). The result of the transformations in Figures 2 and 3 could not be more different. In this case (Figure 3), the parent drug (hydrocodone) is weakly active (5) but one of its major metabolites is very strong.
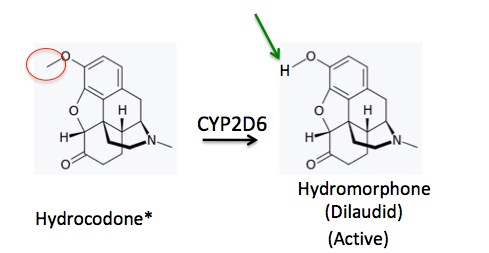
Figure 3. The metabolism of hydrocodone to norhydrocodone. The methyl group (red circle) is removed and replaced by a hydrogen (green arrow). The metabolite, hydromorphone, is stronger than the parent drug.
5. Fentanyl: Better be careful
There is an interesting case study about drug-drug interactions of fentanyl in a 2008 article in The Pharmaceutical Journal. A terminal cancer patient had been taking morphine for his pain but as his disease progressed the morphine was not strong enough. He was switched to a fentanyl patch (50 micrograms) and was doing well until he developed an infection in his chest and was given erythromycin. He then developed a severe fungal infection, probably from the erythromycin and had to be treated with itraconazole, an antifungal drug.
Then it got strange. Within three days he was having severe trouble breathing. Someone at the hospital had his/her thinking cap on because off came the fentanyl patch and his breathing returned to normal. What happened?
Erythromycin and itraconazole are both powerful inhibitors of CYP3A4, the main CYP isoform (6) that is responsible for metabolizing fentanyl. This allowed higher amounts of fentanyl to build up. Like other opioids, fentanyl causes respiratory depression. The patient had been a slow-motion overdose of fentanyl brought about by two drugs that were extraordinarily efficient in preventing his body from "disposing" of it.
6. It's not just drugs. Grapefruit and furanocoumarins
You don't need to take drugs to be at risk from drug-drug interactions. The produce section at your supermarket will do the trick. Grapefruit contains a family of chemicals called furanocoumarins. Like erythromycin and itraconazole, furanocoumarins inhibit the action of CYP3A4, which can cause the elevation of the blood levels in a number of drugs. A 2013 paper entitled Grapefruit–medication interactions: Forbidden fruit or avoidable consequences? lists dozens of drugs that can be affected by grapefruit, for example, cholesterol-lowering drugs (statins), certain antibiotics and antivirals, chemotherapy drugs, and the opioids fentanyl, oxycodone, and oxycodone. And it doesn't take all that much grapefruit (one) or grapefruit juice (about 9 ounces) to cause a " pertinent pharmacokinetic interaction" (7)
Metabolism is often the major roadblock in bringing a drug from pre-clinical studies to the pharmacy. You have just seen a smattering of it. It gets mighty complicated.
NOTES:
(1) CYPs don't just break down substances. They also build new ones, for example, cholesterol.
(2) The name is random. CY = cytochrome, P=pigment, and 450 refers to the wavelength of light (nM) that it absorbs when exposed to carbon monoxide. The numbers and letters that follow classify the CYPs into different families.
(3) Hagelberg NM, Nieminin TH, Saari TI, et al. Voriconazole drastically increases exposure to oral oxycodone. Eur J Clin Pharmacol. 2009;65(3):263-271.
(4) Nieminen TH, Hagelberg NM, Saari TI, et al. Rifampin greatly reduces the plasma concentrations of intravenous and oral oxycodone. Anesthesiology. 2009;110(6):1371-1378.
(5) The hydrocodone vs. hydromorphone question is debatable. Which one is responsible for killing pain? Or is it both. See. Dr. Jeffrey Fudin's 2014 blog about this.
(6) When there are different (and also similar) versions of enzymes that have the same basic structures and functions they can be subdivided into different families called isozymes.
(7) For studies of the impact of grapefruit upon the levels of the blood pressure drug felodipine. Different drug, same concept. See: Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression or Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients.