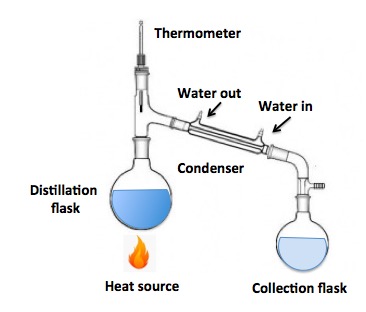
Here's a diagram of a distillation apparatus. It's very simple. The liquid to be purified is placed in a distillation flask. Heat is applied to the flask (1); when the liquid reaches its boiling point the vapor rises up where it passes over a thermometer, enters a water-cooled condenser, which converts the vapor back to a liquid – presumably purer – and it drips into a collecting flask.
For example, here are the boiling points of some common solvents used in chem labs (in ºC):
- Toluene - 111
- Water - 100
- Acetone - 56
Below are two distillation flasks. The flask on the left contains a mixture of toluene and acetone. The two are miscible so they form a clear colorless solution. On the right is a mixture of toluene and water. The two are not miscible; the toluene forms a layer on top of the water, just like gasoline on water.
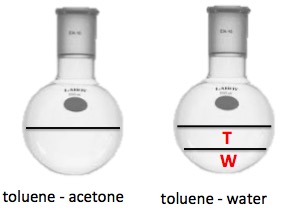
If you distill the toluene-acetone mixture, when the temperature of the mixture reaches 56ºC the acetone, being the lower boiling of the two, will distill out of the mixture and the purified (2) acetone can be collected. Then, as more heat is applied and the temperature in the distillation flask reaches 111ºC the toluene will vaporize and can, likewise, be collected. This mixture behaves just as you'd expect it to.
If you try the same experiment with toluene-water something very different happens. When the temperature reaches 92ºC a messy mixture of toluene and water will start to distill. Huh? How can a mixture of two liquids with boiling points of 100 and 111 possibly distill at a temperature that is lower than either?
You don't really want to know the precise answer because it involves a discussion of the vapor pressure (3) of liquids, a subject (included in physical chemistry - Ewwww) that mercifully exited my life once I got out of grad school. Fortunately, there is a simple explanation. The difference between the two examples can be explained by solubility (miscibility), or in this case, the lack of it.
When a mixture of two miscible liquids is distilled each will vaporize at its own boiling point. But when a mixture of two immiscible liquids is distilled, the vapor pressures of the separate liquids combine; each liquid "helps" the other vaporize. The process of using water to distill a higher boiling immiscible liquid at a lower temperature is called steam distillation (4). In a sense, the water helps "carry over" the other liquid, just what is happening in a vaping device.
Steam distillation will very likely be found to be the cause of some (perhaps even all) of the bizarre cases of serious lung damages that are popping up in the news, and the timing of this new disease is unlikely to be a coincidence.
WHY NOW?
People have smoked billions of e-cigarettes without sustaining the kind of lung damage that we're seeing now. The difference now is that vaping devices are being used to deliver marijuana products, specifically THC and CBD oil. Although this riddle has not been officially solved, an organic chemist pretty much knows the answer – solubility and boiling point.
Chemical Solubility in water Boiling point (ºC)
THC 2.8 mg/liter 200
CBD oil 0.02 mg/liter 464
Looking back at the toluene-water example, it is now reasonable to predict that water in the device would aid in the delivery of THC and CBD – two high-boiling, water-insoluble lipophilic (oil-loving) oils and that these chemicals would be delivered to the lungs as oily droplets, not smoke, as one would get from a joint or cigarette.
It is scientifically logical and also probably that this new and unusual lung condition is a result of more widespread use of marijuana products by a different delivery system. But why hasn't this happened (at least in the past) with nicotine in e-cigarettes?
The situation is different with nicotine; it is completely miscible with water, so the presence of water won't lower its boiling point. Perhaps more important. nicotine is hydrophilic (water-loving) so it won't form insoluble droplets of oil in the lung upon inhalation; it will dissolve and be absorbed or dispersed. The deposition of water-insoluble oils in the lungs is the likely cause of the damage.
OIL AND WATER DON'T MIX. NEITHER DO OIL AND LUNGS
Since marijuana chemicals are not soluble in water other chemicals have been added in an attempt to increase solubility Bad move. One of these additives is vitamin E acetate, which boils at >300°C. This has made matters worse – something that my colleague Dr. Alex Berezow discusses in his companion piece Everything Goes To Pot: Myths Are Driving FDA, CDC Vaping Policy.
BOTTOM LINE
Although no official cause of lung damage has been established, it is likely that the lung damage is caused not by manufactured e-cigarettes, but rather, vaping devices which have been loaded with marijuana chemicals or other normally-harmless chemicals, like vitamin E acetate. Dr. Berezow will explain the rest.
NOTES:
(1) No, Bunsen burners are not used anymore. Open flames in the lab are a no-no.
(2) Simple distillation isn't a great way to separate two liquids. In theory, both the solvents should come out pure, but there is almost always cross-contamination. To prevent this a procedure called fractional distillation is used. Don't ask.
(3) "The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); that is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container." I warned you. Source: Purdue University
(4) Steam distillation is especially useful when the chemical to be distilled has a high boiling point. High-boiling liquids frequently decompose before the temperature in the distillation flask reaches the boiling point.



