
In early April I wrote about a potential coronavirus drug that could be given in pill form. This would automatically vault N-hydroxycytidine (NHC) into the lead in the race to find better anti-coronavirus treatments while the world waits for a vaccine, which may or may not work. NHC inhibits viral replication by the same mechanism as remdesivir (and other antiviral drugs) but is orally active (can be given in pill form). More or less. (More on that later.)
NHC, like a number of other antiviral drugs, is a chain-terminating nucleoside (a nitrogenous base connected to a sugar - ribose or deoxyribose) analog that acts by binding strongly to coronavirus RNA polymerase – the enzyme that builds strands of new viral RNA. In doing so, the drug "tricks" the enzyme into incorporating the NHC rather than adenosine (one of the four nucleosides that make up RNA). And then some.
Unfortunately for you dear readers, the way this works is WAY too cool to ignore, so I must regretfully inform you that it is time for:

For those of you who start dry heaving when I do a TDCLFH®, I suggest you leave now. But, if you're cooped up somewhere and you have absolutely nothing else to do, this is some seriously fascinating chemistry, complete with pretty pictures.
First, you need to know that NHC can exist in two interchangeable forms called tautomers. (Figure 1)
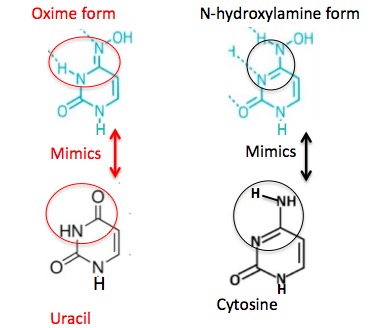
Figure 1. The two forms of NHC. (Left) The oxime form (red circle) mimics the nucleobase uracil. (Right) The N-hydroxylamine form (black oval) mimics cytosine. These are not different chemicals; you cannot isolate one or the other and put it in a bottle. Instead, the NHC molecule can rapidly "switch" between the two forms (called tautomers), so in a solution, both species will exist. Image: C&E News
SO WHAT?
Here's the cool part. Each tautomer mimics a different nucleoside – something I've never seen before. As I mentioned above, the way nucleoside analogs work is by binding to the RNA polymerase enzyme in place of the "proper" nucleoside, which causes the newly-forming RNA chain to stop. In the case of NHC you get two bangs for your buck. Each tautomer mimics a different nucleoside, so one molecule can screw up the viral RNA synthesis in two different ways (Figure 2).
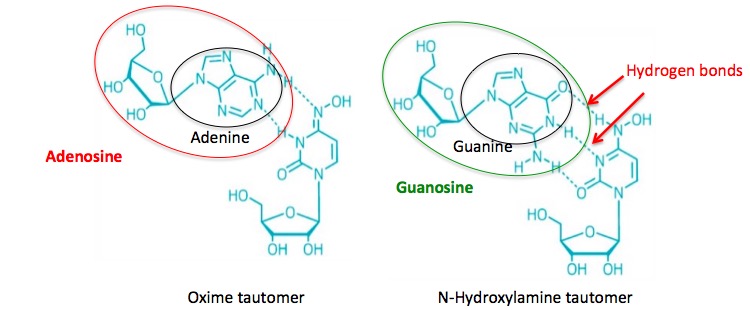
Figure 2. DNA and RNA are held together by hydrogen bonding - a weak bond between hydrogen and nitrogen or oxygen (hatch turquoise lines). For reasons you don't want to know, the hydrogen bonds fit together better (configuration) between adenine and uracil and between guanine and cytosine (this is called pairing) (2). This means that the oxime tautomer can replace adenosine in the growing RNA chain while the N-hydroxylamine tautomer can replace guanosine. Either of these events will stop the chain from growing. One molecule in two different forms is competing with two different nucleosides. For nerds chemists that's as good as it gets.
(You are now excused from the dreaded chemistry lesson from hell®). You have suffered sufficiently.
WHY THE INTEREST IN NHC?
The utility of remdesivir is still unclear. It has some benefit for patients with severe COVID, but this isn't what the doctor ordered. Antiviral drugs work better if given earlier - when the infection begins. We need a drug to keep people out of hospitals much more than one to treat them once they're in the ICU. Remdesivir isn't the answer; the drug is difficult to synthesize and must be given intravenously. That's why we need a pill (See We Need A Remdesivir Pill. Badly.)
NHC IS THAT PILL. SORT OF.
NHC, aka EIDD-1931 (2) is easy to make and is a pill, but it doesn't work; the drug is not well-absorbed, so it doesn't get into the bloodstream. But this problem was rather easily solved (See Remdesivir Is IV-Only. Here Are Some Ways To Fix That using a pro-drug approach. (Figure 3)
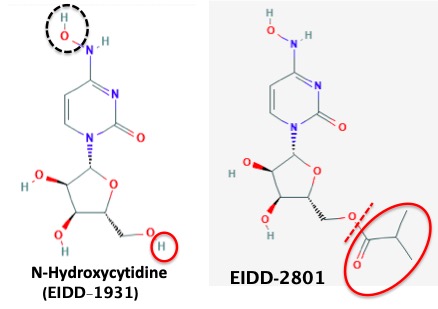
Figure 3. NHC (EIDD-1931) doesn't have the chemical properties required for oral absorption. The drug is too hydrophilic (water-loving, water-soluble) because of the three hydroxyl groups on the ribose ring. For drugs to be orally active they (usually) need to have a balance of water and oil solubility. This was accomplished by converting one of the hydroxyl groups to an ester (red oval), resulting in EIDD-2801, which is absorbed into the blood. Enzymes in the blood then remove the ester, resulting in a significant concentration of NHC in the blood. This is a standard pro-drug approach, which has worked well to solve the same problem for other poorly-absorbed drugs. (3)
NOW WHAT?
Merck, which has been notably absent in the coronavirus arena obviously saw something it liked. The company announced that it will collaborate with Ridgeback Biotherapeutics, a small biotech in Miami, to develop EIDD-2801.
“Since the start of the COVID-19 pandemic we have worked closely with our network of esteemed collaborators to advance EIDD-2801 into the clinic...This agreement with Merck, a leader in infectious disease therapeutics, positions us to harness the full potential of EIDD-2801 and, if approved, deliver it to the patients that need it globally.”
Wendy Holman, CEO, Ridgeback Biotherapeutics
“Clinical evaluation of EIDD-2801 in COVID-19 patients is just beginning, now that phase 1 studies have demonstrated that the compound is well-tolerated. Since preclinical studies demonstrate that EIDD-2801 has potent antiviral properties against multiple coronavirus strains including SARS-CoV-2, we are eager to advance the next phase of clinical studies as rapidly and responsibly as possible.”
Dr. Roger M. Perlmutter, president, Merck Research Laboratories
PREDICTION
I've written many times that you have to be out of your mind to predict whether a drug will make it through clinical trials into the pharmacy. Nonetheless, I'll give it a shot.
EIDD-2801 seems to have everything going for it. It inhibits viral replication in cultured cells, operating by a well-known mechanism (RNA polymerase inhibition), which has worked for numerous antiviral drugs in multiple therapeutic areas. It can be taken orally (a pill), which means that it can be used at the first sign of infection. It is easy to synthesize. And Merck didn't become Merck by making bad decisions.
I predict (and hope) that EIDD-2801 will be the first successful oral therapy for COVID and that it will be a game-changer. Clinical trials began in the UK in April. The drug seems to be well-tolerated. Stay tuned and keep your fingers and toes crossed.
NOTES:
1. Adenine (a nitrogenous base) attached to ribose is called adenosine (a nucleoside). The same goes for the other three bases that make up RNA and their corresponding nucleosides. When guanine is attached to ribose it's called guanosine. Uracil becomes uridine. Cytosine becomes cytidine. Madness. No wonder people hate chemistry.
2. EIDD is short for the Emory Institute for Drug Development. Emory University is a very big name when it comes to antiviral drugs. Emtricitabine, one of the two AIDS drugs that make Truvada was invented at Emory. Chemistry professor Dennis Liotta, who is widely known for his work in this area, was one of the discoverers of emtricitabine.
3. In this case, it's a bit more complicated. NHC was absorbed in mice and dogs, but in monkeys, the hydroxyl group that was later converted into the ester was phosphorylated within the cells of the gut. Phosphates do not readily cross cell membranes, so the drug was stuck inside the cells in the monkey's stomachs. The esterification took care of this problem. Very clever.



