This article was originally published at Geopolitical Futures. The original is here.
The American pharmaceutical firm Pfizer, in collaboration with German firm BioNTech, surprised the world when it announced that its coronavirus vaccine showed 90 percent efficacy in preventing COVID-19. Days later, another American firm called Moderna announced that its vaccine was nearly 95 percent effective. And shortly after that, AstraZeneca announced that its vaccine was 62 percent to 90 percent effective. The U.S. Food and Drug Administration dictates that vaccines be at least 50 percent effective to earn emergency use authorization, and most observers weren’t expecting vaccine candidates to perform much better than that. The reported results, therefore, were a pleasant surprise that excited governments and markets alike.
The magnitude of this accomplishment cannot be overstated. Typically, the timeline from inception to regulatory approval of a new drug is about 10 years. After receiving approval, pharmaceutical firms then prepare for mass manufacturing, which itself could take another decade. However, thanks to a combination of factors – government programs like Operation Warp Speed, expedited regulatory approval and unprecedented global cooperation – the first batches of a COVID-19 vaccine from a trustworthy source will be delivered in less than a year. (China and Russia also claim to have created vaccines, but insufficient data and transparency make most Western scientists skeptical of their efficacy and safety.)
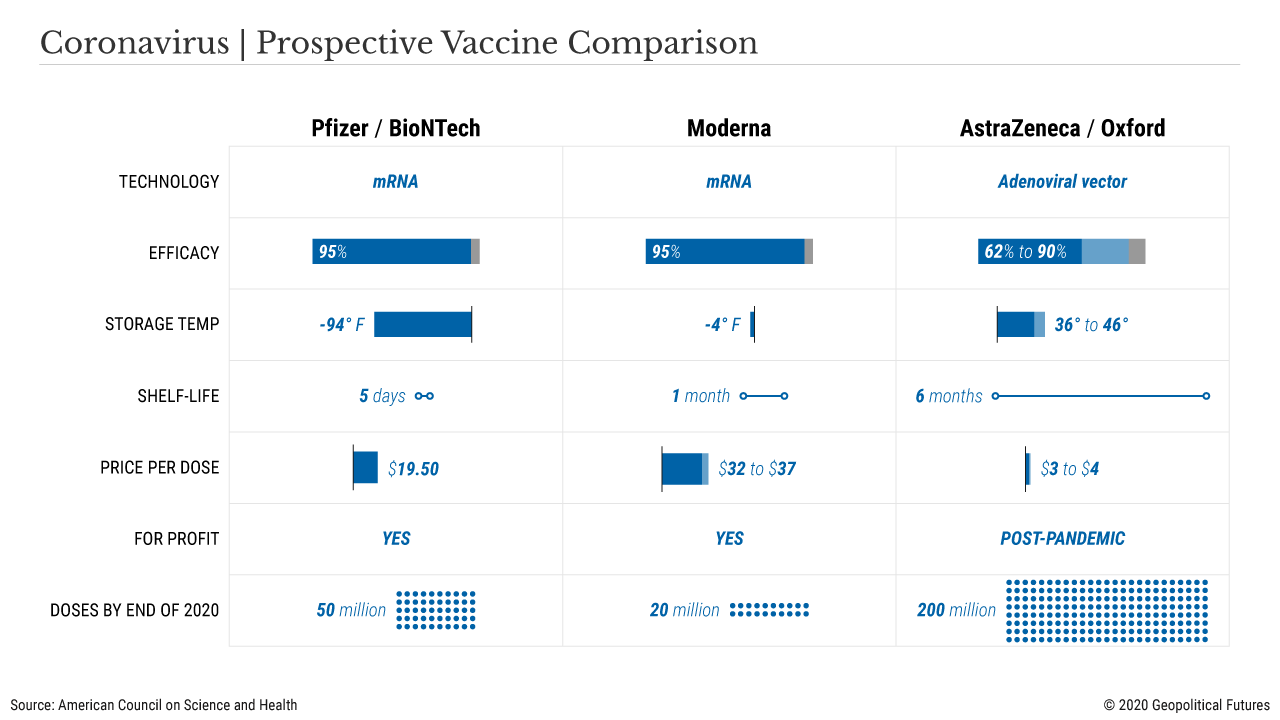
Even so, public excitement is premature. There are months to go before the vast majority of people, including Americans, can expect to receive the jab in their arms. The immediate hurdle is obtaining FDA approval. Though approval should take roughly three weeks as government experts pore over the data, the process could take longer. According to Dr. Henry Miller, a fellow at the Pacific Research Institute and the founding director of the FDA’s Office of Biotechnology, the FDA has concerns about consistency in the production of the vaccine. In other words, the FDA wants to know if companies can manufacture batch after batch that meets certain quality control measures, such as potency and purity. By the end of December, Moderna plans to have 20 million doses, Pfizer 50 million doses, and AstraZeneca 200 million doses. So, some people should be receiving jabs before the end of 2020. (The regimen requires two shots one month apart, which means the number of people immunized is half the number of doses.) By the end of 2021, there should be billions of doses available from all companies combined.
But manufacturing is just one headache. Another is distribution, which is actually a two-fold problem: (1) Moderna’s vaccine must be kept frozen during long-term storage and shipment, but Pfizer’s vaccine must be kept at a whopping -94 degrees Fahrenheit (-70 degrees Celsius) during shipment; and (2) It’s neither ethically nor strategically clear who should receive the vaccines first.
The Distribution Dilemma
The reason your local grocery store contains relatively fresh food from the other side of the planet is because of something known as the “cold chain,” a series of refrigerated containers that allow perishable food to be shipped without spoiling. Many drugs and especially vaccines require the same thing. However, Pfizer’s vaccine, which consists of an unstable information-containing molecule called RNA encased within an equally unstable bubble of fat, requires storage at -94 F (-70 C). Generally, only research laboratories possess deep freezers that cold; pharmacies and hospitals do not. Pfizer’s solution is to provide special containers that can be packed with dry ice to maintain the requisite temperature, but once the vaccine has been removed and placed inside a regular refrigerator, the shelf life is five days. The Moderna vaccine can be stored and shipped at -4 F and has a shelf-life of 30 days in regulator refrigerators, so it poses a much smaller logistical challenge. AstraZeneca’s vaccine is the easiest to distribute, since it can be shipped at the regular refrigeration temperature of 36-46 F and kept on the shelf for six months.
The question of who gets the vaccine first will play out initially at the international level. Countries that have made purchase orders will be the first to receive them. For example, according to Mint, the European Union has secured 200 million doses (with an option for 100 million more) for Pfizer’s vaccine, Japan 120 million doses, the United States 100 million doses (with an option for 500 million more), and the U.K. 30 million doses. If all options are exercised, Pfizer simply cannot meet that demand even by the end of 2021, which could mean that rich countries will fight among themselves over who gets what batch when. The U.S. already has a tense relationship with Europe, and a fight over vaccine batches would put more stress on a fragile trans-Atlantic relationship, especially since the country that receives the most vaccine doses has a likelier chance of improving its economy the quickest. Because Moderna, AstraZeneca and other companies will also produce vaccines, these tensions can be eased somewhat, though whichever country serves as a company’s “home” likely will be under substantial pressure to deliver vaccines to compatriots first. This latter point is complicated by the fact that some of these companies are multinational entities and have “homes” in several countries around the globe.
While rich countries duke it out, the rest of the world will have to wait. Money doesn’t solve everything. The logistical challenges posed by the cold chain make it nearly impossible to deliver a vaccine to a region with poor infrastructure and unreliable electricity. Gaps in the cold chain – that is, periods in which the vaccine is not stored at the proper temperature – would destroy the vaccine. (This is to say nothing of criminal activity. Everyone is a potential target of criminal enterprises that offer fake vaccines, of course, but poorer countries are most susceptible since delivery of legitimate vaccines may be months if not years away.)
Internally, countries will be faced with domestic political concerns over vaccine allocation. In the United States, there is tension between federal agencies such as the Centers for Disease Control and Prevention and state governments over the number of doses each state will receive. The incoming Biden administration will be under tremendous pressure to deliver vaccines to Americans first, regardless of any contractual obligations that companies may have. If necessary, the president and Congress may get involved, overriding pharmaceutical companies’ contracts in the name of national security.
After that, there are ethical and strategic decisions to be made over who receives the vaccine first. The state of Washington, for example, has proposed three phases of distribution: First, frontline health care providers, first responders, and vulnerable populations (such as those with underlying health conditions or residents of long-term care facilities); second, the general community; and third, filling any “gaps” in vaccine access (such as in poorer communities). It is likely that many other states will employ a similar strategy.
There’s also the matter of vaccine efficacy. Simply put, some vaccines are better than others. Remember that the vaccines made by Pfizer and Moderna are 95 percent effective, but AstraZeneca’s is merely 62 percent to 90 percent effective. Who gets the less effective vaccine? And who makes that decision?
The Amazing Race
The poorest nations are likeliest to get a coronavirus vaccine last. But there’s room for optimism as many other companies will continue developing coronavirus vaccines. Most, like the one made by AstraZeneca, will not require the extreme cold chain that Pfizer’s vaccine needs. In addition, AstraZeneca has pledged to forgo profits until the pandemic is over. The company is also working with nongovernmental organizations like the Bill & Melinda Gates Foundation to provide vaccines to developing countries.
The question about global vaccine distribution has therefore shifted from if to when. But will the vaccine(s) come in time to prevent further economic damage and social unrest? Vaccine delays could create or aggravate default risks in poorer countries, with financial contagion spreading to richer countries. Many people are no longer willing to tolerate lockdowns. All across Europe citizens are protesting additional safety measures, with demonstrations in Italy turning violent last week.
Ancillary effects of lockdowns have created other grievances against governments too. In many places, families and spouses have been separated because of border closures and arbitrary immigration policies. In Indonesia, for example, binational unmarried couples are not allowed to reunite, but elderly foreigners are allowed in as tourists despite the fact that elderly people are the likeliest to die from coronavirus. These policies have resulted in the Indonesian government being inundated with complaints by angry citizens. They reveal the tension between concerns over public health, the economy and the social fabric, and it’s not clear that they can be improved until a vaccine is fully deployed.
Indeed, few societies are willing to control the virus at the cost of ripping the social fabric. The coronavirus has revealed an immense tension between the economic and social pillars of our society. Governments have no good options. Some will be tempted to reimpose lockdowns, justifying them with the claim that they will be eased as soon as a vaccine is available. But this is a false reassurance. For citizens of rich countries, widespread vaccination is still months away. For citizens of poor countries, widespread vaccination may be years away. A restless public is not going to tolerate indefinite lockdowns through 2021, and governments that try to impose them should be prepared for civil unrest.
© 2020 Geopolitical Futures. Republished with permission.




