Fructose is a much-maligned and misunderstood ingredient in foods. To understand why we must look at its history, structure, functionality, where it occurs naturally, how it is made synthetically, and compare fructose to other sugars in foods.
Simple Sugar Structures Affect Sweetness
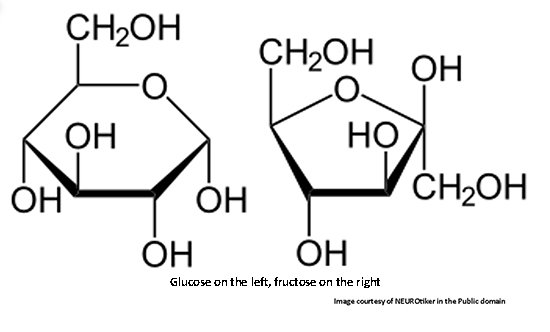
Here are the structures of the naturally-occurring sugars in our discussion:
Glucose on the left and fructose on the right. They are called simple sugars or monosaccharides. Both have the chemical formula of C6H12O6. Glucose and fructose are isomers, meaning they have the same number of ‘chemical ingredients’ - carbon (C), oxygen (O), and hydrogen (H), but they are arranged differently. (The carbons are not shown in projections but are implied by bends.)
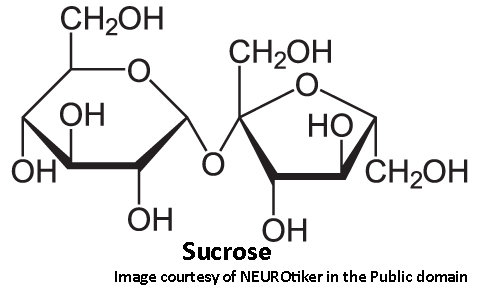 Because of double-bonded oxygen, glucose forms a 6-sided ring while fructose forms a 5-sided ring. This gives them different functional properties. Most notably, it affects the ‘sweetness’ of each. The relative sweetness of various sugars is determined using sucrose as the standard. Sucrose is a disaccharide consisting of two simple sugars, glucose and fructose, bound together.
Because of double-bonded oxygen, glucose forms a 6-sided ring while fructose forms a 5-sided ring. This gives them different functional properties. Most notably, it affects the ‘sweetness’ of each. The relative sweetness of various sugars is determined using sucrose as the standard. Sucrose is a disaccharide consisting of two simple sugars, glucose and fructose, bound together.
Sucrose, being the standard, has an arbitrary sweetness of 1.0. Glucose has a sweetness of only 0.56, meaning it is only about half as sweet as sucrose. Fructose has a relative sweetness of 1.3 - 30% sweeter than sucrose and much sweeter than glucose. This is one reason why fructose is a desirable sweetener; you can use less to get the same toothsome effect.
Sugar Sources
Fructose, glucose, and sucrose are all naturally-occurring sugars. Fructose is also called fruit sugar because it is found in many fruits as well as honey, flowers, and most root vegetables—ditto for sucrose. Glucose is also found in plants and most algae. It is created during photosynthesis. Whether from sugar cane, sugar beets, honey, fruit, or tree sap, these sugars have been consumed over the ages by humans as a rich source of calories and as flavor enhancers.
The white granules in the sugar bowl.
One form of sucrose is table sugar. While sucrose was the standard for sweeteners, it had issues as an ingredient. It posed technological problems in beverages such as sodas because it was granular and had to be dissolved in water before use. It was also unstable in the acidic sodas, breaking up and changing the sweetness and flavors of the drinks. In the mid-twentieth century, most sucrose came from sugar cane grown in the tropics. Political instability in those regions would cause the price and availability of sugar cane to vary widely.
 High Fructose Corn Syrup (HFCS) was developed in the 1950s as a liquid alternative to granular sucrose in foods and beverages. It is a liquid, stable in acidic conditions, and easy to deliver and mix into foods and drinks. HFCS comes from corn, grown domestically in the United States, an abundant renewable resource.
High Fructose Corn Syrup (HFCS) was developed in the 1950s as a liquid alternative to granular sucrose in foods and beverages. It is a liquid, stable in acidic conditions, and easy to deliver and mix into foods and drinks. HFCS comes from corn, grown domestically in the United States, an abundant renewable resource.
History of High Fructose Corn Syrup
HFCS is made from cornstarch, the result of the milling of corn – the same process that gives us flour from wheat. The cornstarch is heated and treated with enzymes to convert some of the glucose to fructose, increasing its sweetness. It is then blended with pure glucose syrup to create three different concentrations of HCFS. Whether from a processing plant converted from corn, fruit juice, or tree sap, glucose is glucose, and fructose is fructose. They are the same on a molecular level.
In the late 1960s, HFCS experienced tremendous acceptance and use in the food industry, making it one of the most successful ingredients in modern food history. HFCS was an established and little-noted food ingredient on many labels until 2004, when researchers published an article naming HFCS as a causative factor for the climbing obesity rates between 1960 and 2000.
The researchers pointed out that the digestion, absorption, and metabolism of fructose differed from glucose. Unlike glucose, fructose does not stimulate insulin secretion or spur leptin production, both factors in regulating the amount of food eaten and maintaining body weight. They hypothesized that fructose might cause increased calorie intake leading to weight gain. They concluded with this general but obvious statement about sugars, “…. Calorically sweetened beverages may enhance caloric overconsumption.”
Since it touches our lives at least three times a day and is a cornerstone of health and wellness, food was a major topic of internet bloggers, social media, and purveyors of alternative foods and supplements. The problem with HFCS was one of perception. Some sources and processes are perceived by consumers as more ‘natural’ and therefore healthier than others. ‘High’ fructose meant ‘high’ calorie, something to be avoided in the obesity epidemic. It was a regrettable choice to use high in the ingredient name to distinguish it from glucose-only corn syrup. The ratio of fructose to glucose is 1:1 in HFCS, similar to honey, sucrose, fruits, and fruit juices. Historically, when HFCS replaced sucrose, there was no increase in fructose in food formulations.
“What’s in a name? That which we call a rose by any other name would smell as sweet.”
We might call high fructose by yet another name, honey, which is just as sweet. There are two types of commonly-used HFCS, named HFCS-42 and HFCS-55. The numeral designation means that they contain either 42 or 55% fructose. Honey contains 49% fructose, 7% more fructose than HFCS-42. However, most consumers will insist that honey is natural and good; but HFCS is processed and therefore bad. Chemists and nutritionists will maintain that the two are comparable. Rather than demonize one ingredient due to its unfortunate technical name, better to prevent or control obesity by reducing calories from all sugars, regardless of the name or source.