I recently wrote that PROP President Andrew Kolodny's off-the-cuff remark about controlling the latest and arguably worst drug – xylazine, aka Tranq – by making it a DEA-scheduled drug was both naive and badly flawed.
"For sure, you would not see xylazine all over the place if it was scheduled."
Andrew Kolodny in MedPage Today, "Will Xylazine Become a Controlled Substance?" 2/23/23
I very much doubt it. After all, heroin and fentanyl are both scheduled drugs, and it sure hasn't put a dent in the massive amounts of these, especially the latter, from entering the US. So, why should slapping an essentially meaningless label on a veterinarian tranquilizer prevent it from infiltrating the already-infiltrated illicit fentanyl supply (90% of fentanyl samples in Philadelphia now contain xylazine) make a difference? Kolodny's answer is quite revealing:
"If it were scheduled, distributors would have to have a suspicious order monitoring system. It's a DEA [requirement] that would tell you, for example, your Philadelphia region, or a particular veterinary practice you're distributing to, are outliers and you can't continue to fill that order. You have to report them."
In other words, it would seem that Kolodny wants to extend the war against physicians (opioids) to veterinarians and try to "control" yet another uncontrollable drug. As if giving the DEA the power to look over vets' shoulders will make Tranq magically disappear from the streets of Philadelphia. But it's all part of the same twisted mindset: pressuring caregivers to stop prescribing "bad" drugs while at the same time punishing those who need those drugs.
If physicians stop prescribing opioids altogether – not as absurd as you'd think – the DEA, perhaps not having enough to justify its budget, could bust onto the set of Dr. Pol – while he has his entire arm inserted into a cow's reproductive apparatus – and count xylazine bottles. (And don't think I'm kidding about the arm thing.)
Other reasons scheduling Tranq won't work
Even if all the xylazine in every vet's office on earth was being confiscated it won't make a difference. Here's one reason. You can buy it online from China for ten bucks per kilo. And plenty of other places, but its more expensive.
Or you can make it yourself. Very easily.
Organic chemistry will defeat any attempt to control xylazine
Something that is less obvious is that you could incinerate every atom of xylazine in the world, and if there's a demand for it, illicit xylazine – or, something worse, perhaps a xylazine analog – will cheerfully take its place to meet market demand. To understand how simple it is to make the stuff, let's look at an old paper where xylazine (and many other compounds) were synthesized and investigated as potential hypotensive drugs.
Did I hear the word "synthesis?" You all know what this means. Buckle up, boys and girls! This article contains chemistry, so it's time for another hideous chapter of...

Our TDCLFH® hosts Steve (left) and Irving seem to be a bit cross with each other. Perhaps too much time (eternity) together in a place with no air conditioning.
A (mercifully brief) look at the literature
If the title of this 1975 paper, which was published in the Journal of Medicinal Chemistry (JMC), doesn't have you drooling in anticipation, nothing will:

Pay attention to one word – the last one – because xylidine, as you'll see shortly, is a close analog of clonidine, a vasoconstrictor. The prevailing theory about why xylazine causes grotesque skin lesions <------ (Don't look. Consider yourself warned) is that its vasoconstrictive properties cause localized areas of tissue with low oxygen, and this causes skin death.
You can see how they are chemically similar.
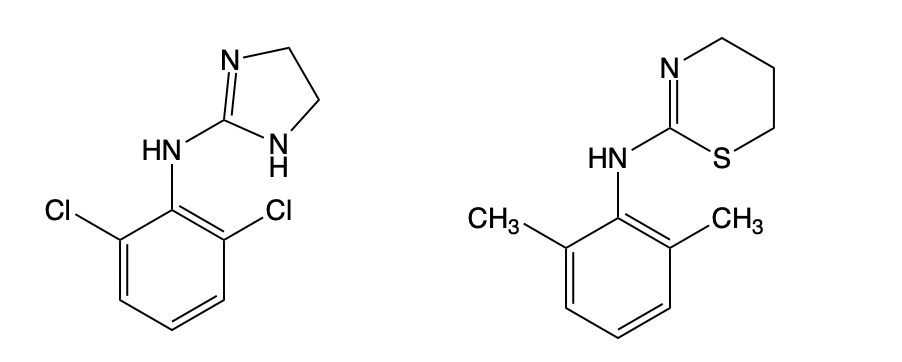
Figure 1. The chemical structures of clonidine (R) and xylazine (L) It is not at all surprising that they both possess vasoconstrictive properties.
The JMC paper describes the synthesis and biological properties of dozens of chemical compounds in 11 different (but similar) chemical classes. Studying structure-activity relationships (SAR) – how changes in structure affect biological properties – requires that chemists make dozens, more often hundreds, of related analogs. Although SAR is an old-fashioned approach to drug discovery, it is still used today. Below is a living nightmare one of the tables in the 10-page paper. Take a Klonipin first.
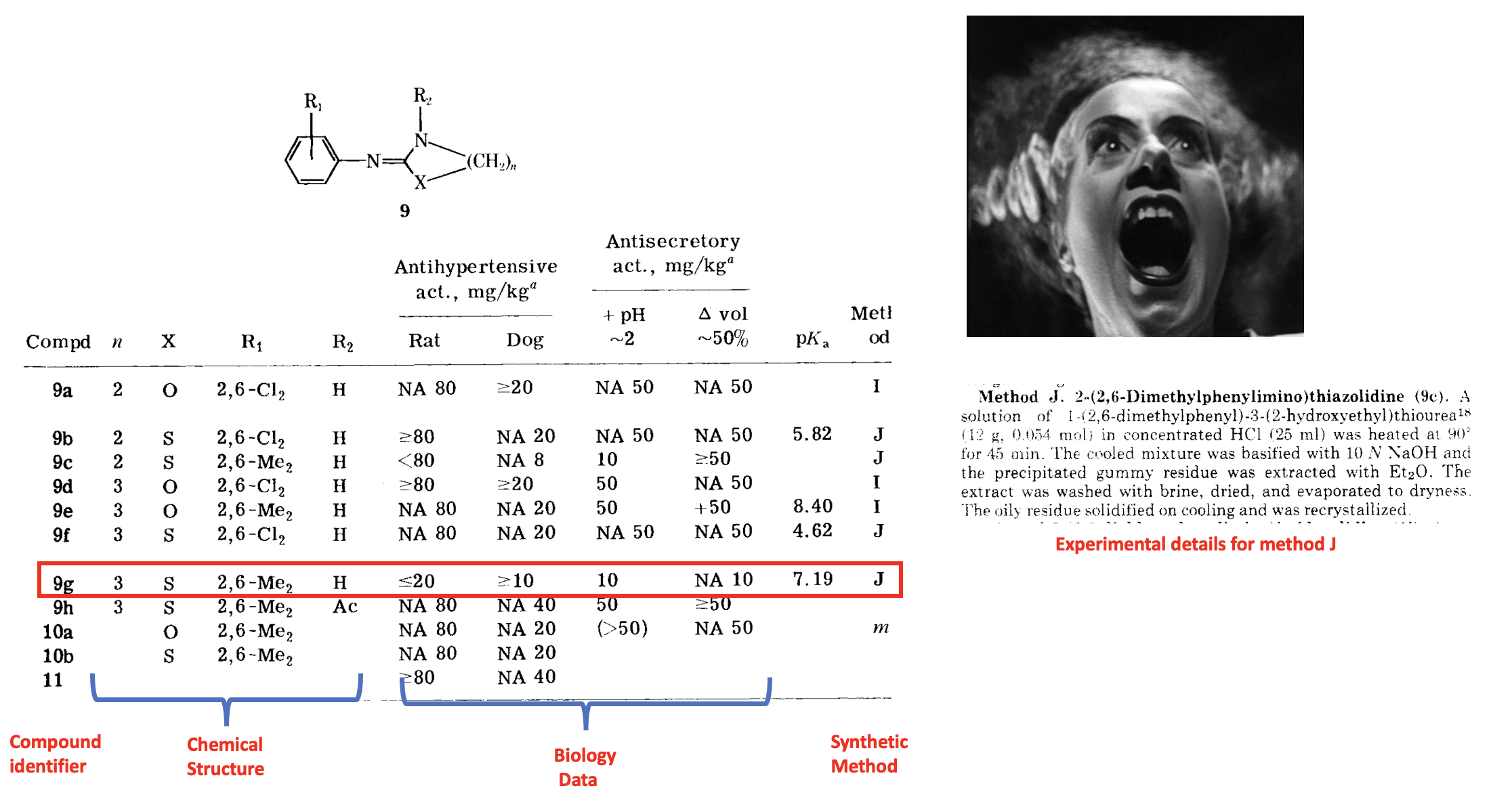
Table 1 appears to be nothing special (except, perhaps, a new CIA-sanctioned torture method), but taking the time (plenty) to comb through the damn thing revealed a compound that was nothing special at the time but it is now. The compound, harmlessly denoted by 9g (red box), is xylazine, although you'd need to be an organic chemist to know this. (Right) An image of the really simple procedure that the authors used to synthesize xylazine.
Free image: Pere Uba, Flicker
As I've written before, the synthesis of fentanyl is relatively simple. But xylazine could be made in a broom closet by a dyslexic Rottweiler. So, the real problem in controlling the drug (or any others in the family) is that the same chemistry applies to xylazine analogs. As you'll see below, there is an infinite number of these, 99+% of which don't even exist, that can also easily be made. Here's how.
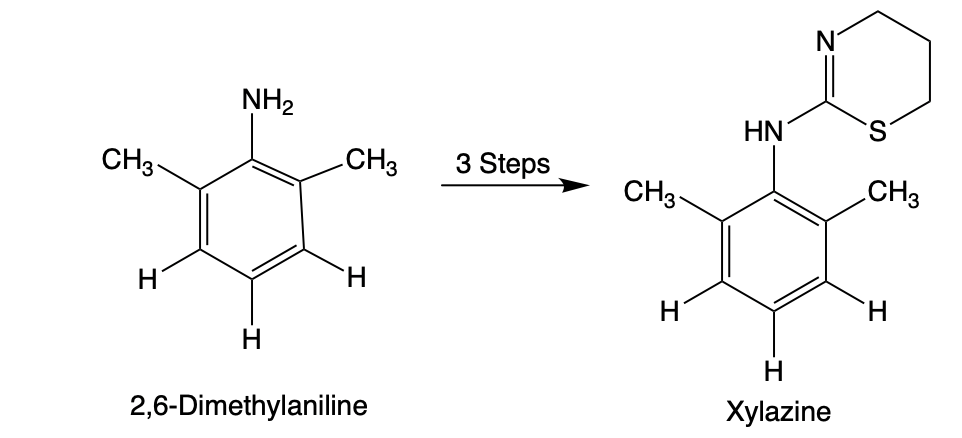
Figure 2. 2,6-Dimethylaniline is a very common and unrestricted chemical [QUIZ - WHAT OTHER MEDICINE IS IT USED FOR?] that can be purchased from dozens of suppliers. Using a simple 3-step synthetic sequence that I previously described, it can easily be converted to xylazine. But here's where the power of synthetic organic chemistry is revealed.
Analoging - taking it to the next level
In Figure 3 (below) I have drawn (1) a variation of the structure of 2,6-dimethylaniline (Left). where the amino group must contain two hydrogen atoms, and the 2 and 6 positions (red) must be connected to a carbon atom. But I've now allowed all the other hydrogen atoms of 2,6-dimethylaniline to be replaced any atom (A) (2). A substructure search (3) of the ChemSpider database of commercially available chemicals for chemicals you can buy that fit the criteria for query structure X gave more than 10,000 chemicals, so one could theoretically make more than 10,000 xylazine analogs.
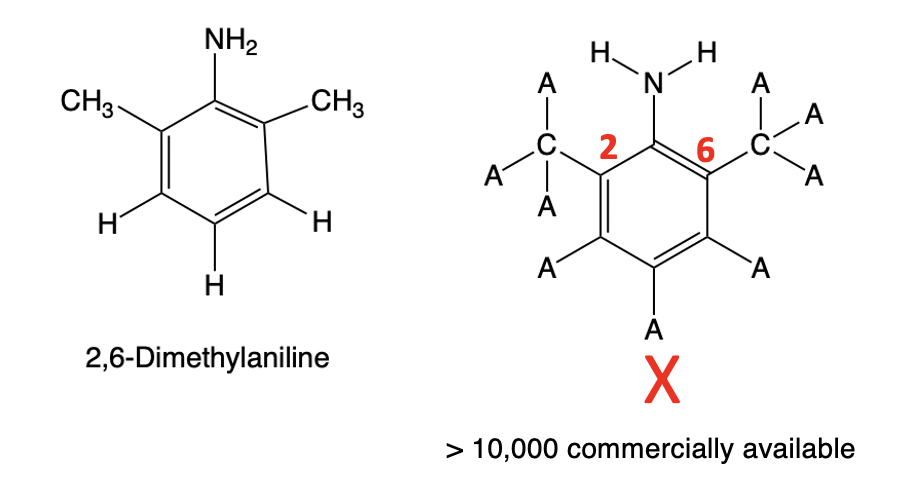
Figure 3. Xylazine and a substructure.
But it gets worse
In query structure X (above), I specified that the atoms bound to the 2 and 6 positions (red numbers) must be carbon. This is an arbitrary choice. Below is a broader query structure where any atom or group of atoms can be attached to the aminobenzene (aniline) ring. There are an infinite number of possible chemical compounds that fit this formula, which can theoretically be made into an infinite number of xylazine analogs.
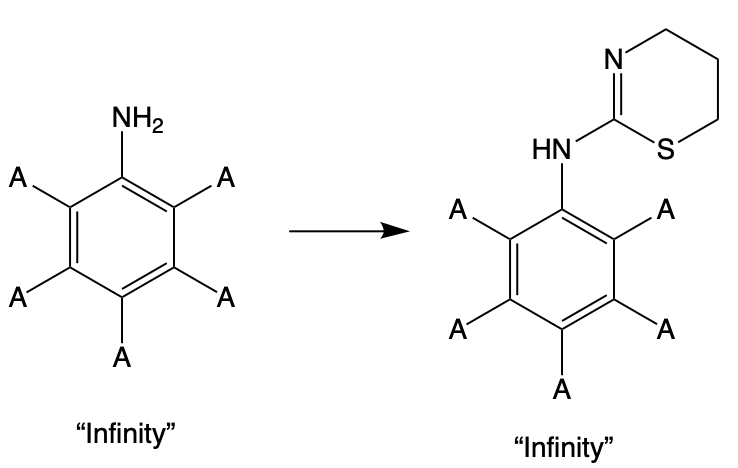
Of course, this won't happen. If faced with such a job, chemists will buy a few dozen or so close varieties of 2,6-dimethylaniline, knowing that small changes will be much more likely to produce new drugs with xylazine-like properties. Of course, some of these have already been made (Table 1), but this barely scratches the surface of possible xylazine analogs that could be made should xylazine itself become unavailable.
A critical question is whether these analogs are better or worse (whatever that means). Safer or more dangerous? We'll only know when they start showing up on the street – a macabre clinical trial if ever there was one. (4)
Bottom line
Scheduling drugs like xylazine to make them less available hasn't worked with heroin or (especially) fentanyl. There is no reason to expect a different result in this case. Even a mediocre organic chemist will find a way to overcome the ban or shortage of one drug by simply making more of it another similar analog. If only the ignoramuses in charge of our drug policy understood this, perhaps our insane drug laws would be a little less so.
NOTES:
(1) Thanks to Mick Hitchcock for his generous donation that enabled ACSH to purchase ChemDraw so that I can finally draw structures without modifying ones from Wikipedia. Mick, you wanted chemical structures. You got 'em.
(2) Of course, many of the chemicals that make up "atom A" are ridiculous and/or do not exist. Good luck finding a compound where A = plutonium, xenon, or tungsten. In reality, commercially available chemicals will have common elements as "A," such as carbon, oxygen, chlorine, or nitrogen. Don't worry - there will still be plenty of them.
(3) When the structure being searched (the search query) contains multiple possible answers this is called a substructure search. Depending on how the query structure is defined a substructure search can give very few (even zero) "hits" or tens of thousands.
(4) This is conceptually similar to fentanyl analoging, something I wrote about in 2018.




