From a chemist's perspective, perhaps the most intriguing part of Fox's recent interview with Jane Pauley of CBS News was his admission that he may have inadvertently done something that contributed to his development of Parkinson's. Pauley, alluding to a prior comment that Fox made in 2022 during an award ceremony, asked "Is it possible you did some damage?"
His answer:
"Yeah, very possible. ... I mean, there's so many ways that you can, that I could've hurt myself. I could've hit my head. I could've drank too much at a certain developmental period. Most likely I think is, that I was exposed to some kind of chemical. What we say is that genetics loads the gun and environment pulls the trigger."
Why is this quote so intriguing? Because it's happened before, albeit under different circumstances. And the culprit was indeed "some kind of chemical," but it wasn't alcohol or anything in the environment. In 1982 a group of heroin addicts who injected a bad batch of an analog of Demerol developed severe Parkinson's symptoms – in days, not years. The drug, which is called MPPP was contaminated with a highly neurotoxic impurity and the results were both astonishing and horrifying. The incident can be traced back to 1976 when a graduate student made a bad mistake in the lab. There is even a book about the incident, although the author gets the chemistry wrong.

Nope, it was not heroin
Demerol is now rarely used and MPPP – the Demerol analog that did the damage – was certainly not the cause of Fox's disease; this drug is long gone. But it left a legacy that contributed to the understanding of Parkinson's.
###
(Below is an updated version of a similar article I wrote on this topic in 2017.)
Frozen Addicts, Garage Drugs, And Funky Brain Chemistry
In the 1970s chemists in the illicit drug business began exploring a new class of psychoactive drugs, which were structurally related to the synthetic opiate Demerol. The following story is truly one-in-a-million. It encompasses the first "designer drug," (1) the organic chemistry used to make it, and what can (and did) happen when a reaction procedure is not conducted properly. On a "positive" note, the incident did result in a deeper understanding of the molecular mechanism of Parkinson's Disease. It also makes for a fascinating story.
The story began in 1947 when Demerol was discovered at Hoffman-La-Roche. At that time, drug companies were searching for pain-killing drugs that did not have the baggage of standard opioid drugs, such as hydrocodone and oxycodone (2). In fact, desmethylprodine (MPPP), which is considered to be the first designer drug, was also discovered at Roche around that time, but, unlike Demerol, it was never approved.
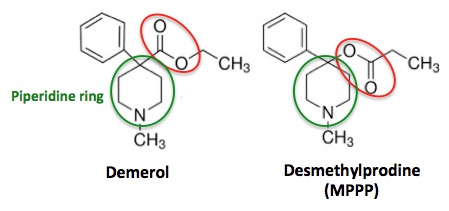
Figure 2. The chemical structures of Demerol (L) and Desmethylprodine (R)
Similar chemicals can act very differently
Even people with little or no chemistry knowledge can see that the two molecules in Figure 1 are nearly identical in structure. The only difference is the functional group (red circle) that is found on the piperidine ring (green circle). In Demerol, the carbonyl group (carbon double bond to oxygen) is attached to the piperidine ring. But in MPPP, an isomer (2) of Demerol, the carbonyl group and oxygen atom are switched, so that oxygen is attached to the piperidine ring. While this may seem like a trivial difference, chemically it is anything but—something that Barry Kidston, a chemistry grad student, would find out the hard way in 1976.

Barry Kidston Photo: Facebook
Things start to go South
Kidston was interested in discovering legal narcotics for his own use. Since MPPP, which has morphine-like properties, was neither approved, nor designated as a Schedule I drug (3), it was perfectly legal to make or use—something that Kidston did for months. Then he got a little sloppy with his chemistry, and the results were disastrous. The mishap was simply a matter of his failure to control the temperature of the MPPP-forming reaction. For organic chemists, the results of this mistake are not only logical but predictable. What happened after that was anything but.
The chemical reaction below shows the final step in Kidston's MPPP synthesis (its precursor, HPMP, is easy to synthesize). It's a common, usually trivial procedure called acylation (Figure 3).
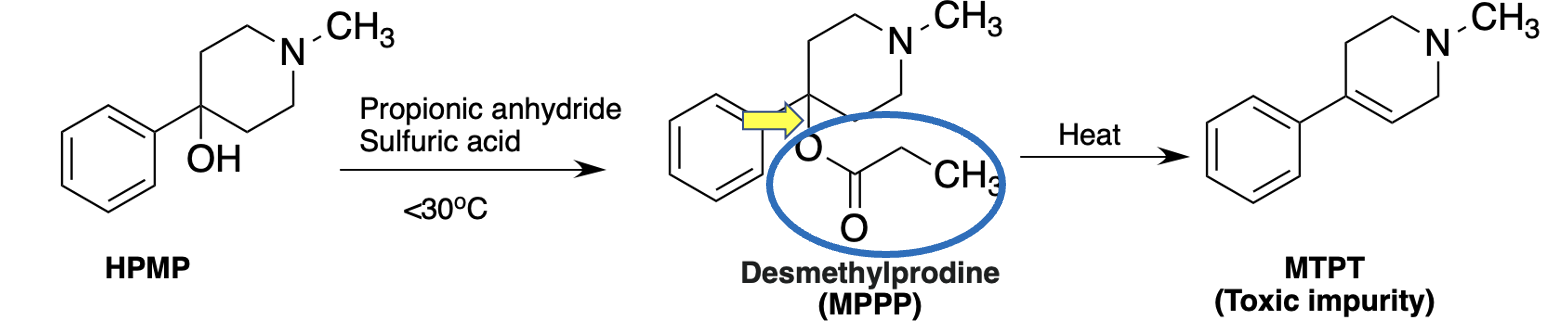
Figure 3. Synthesis of MPPP. When HPMP is reacted with propionic anhydride MPPP, the desired drug, is formed. But if the reaction temperature rises about 30oC some of the MPPP is converted to MPTP, a toxic impurity,
Any organic chemist will tell you that the bond denoted by the yellow arrow is just dying to break, and it doesn't take much to give it its wish. A little extra heat and that bond breaks, and then through a process called elimination, the propionyl group (blue oval) departs and is replaced by a carbon-carbon double bond. Then, instead of pure MPPP, you have the drug plus unwanted MPTP as an impurity.
If you're asking yourself "What's the harm in having a bit of an impurity in there?" the answer is "usually not much." But in this particular case, the impurity did something unprecedented in the brain, which is what makes this story so unique.
MPTP (unfortunately) goes to the brain, which becomes its own worst enemy
Any experienced medicinal chemist will look at the structure of MPTP and know that it is very likely to have the properties that will allow it to cross the blood-brain barrier, which it does. This is when things went very wrong. MPTP itself isn't particularly toxic, but it gets metabolized in the brain to MPP+, a chemical that you do not want in your brain. Unfortunately for Kidston, he was the lab rat who would inadvertently provide the science world with a fascinating, but deadly lesson in neurochemistry. Shown below is the metabolism that is responsible for a previously-unheard of event:
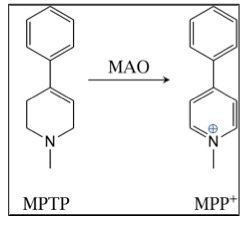
Oxidation of MPTP to MPP+ by monoamine oxidase in the brain
A ubiquitous family of enzymes called monoamine oxidases (MAO) is responsible for the formation and metabolism of multiple neurochemicals. As such, it plays a critical role in the regulation of the central nervous system. In this case, MPTP was a substrate for MAO, which oxidized it to MPP+. Assuming that enzymes can make "mistakes," this is one a doozy because MPP+ is seriously bad news. It is now known that it has a particular fondness for the cells in the region of the brain called the substantia nigra. And once it gets there, all hell breaks loose (4). MPP+ specifically kills the dopaminergic neurons in the substantia nigra, where dopamine is normally made. The absence of dopamine is the hallmark of Parkinson's.
The bad batch of MPPP that Kidston injected into himself resulted in the development of strange symptoms within a few days. He experienced bradykinesia — a severe slowing of movement. It became so bad that Kidston was admitted to a hospital where he was diagnosed with Parkinson's Disease, which is very rare in young people. Nonetheless, Kidston did get Parkinson's — from a single injection of an impure drug.
Doctors tried a variety of neuroleptic drugs with no success and then used L-dopa, which was first tried on Parkinson's patients (with mixed success) in the 1960s. It worked on Kidston, at least for a while. L-dopa loses effectiveness with time. This happened to Kidston, who became severely depressed and died of a cocaine overdose 18 months later.
The link between MPTP and Kidston's Parkinson's Disease was determined by analysis of the residue of the drug that remained on the glassware he used. The impurities were isolated, identified, and tested. Kidston had unknowingly written a chapter on the neurochemistry of Parkinson's Disease at his own expense.
Frozen addicts
The MPP+ story did not end with Kidston. In California in 1982, a cluster of six drug users who had taken "China White" (5) all came down with Parkinson-like symptoms (6). When the drug was analyzed, there it was — MPTP, which was a result of a drug dealer trying to do organic synthesis in his garage. The reason for the term "frozen addicts" could not be more clear:
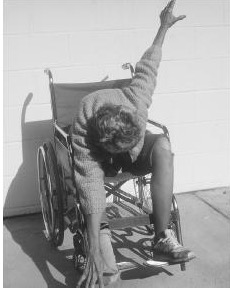
A Frozen Addict from 1982. Source: Neurology Update.
Organic synthesis is both science and art — something that anyone who takes street drugs should consider. It requires both skill and experience to get it right. A drug dealer working in a garage is probably not going to get it right.
Bottom Line
Whatever caused Fox's Parkinson's may or may not have been related to an injury, drinking, or exposure to a chemical or drug (note: he did not say that he took any drugs). We will never know. The stories of Barry Kidston and Fox – only 15 years apart (7) intersect only in that two young men were struck by a terrible disease very early in life. Kidston's disease was clearly self-inflicted and its role in causing Parkinson's has been rigorously proven in animal models. On the other hand, what Fox expressed about the possibility of his Parkinson's being linked to chemical exposure isn't impossible; it's just unlikely (8).
It is more than 47 years since Kidston's blunder and 30 since Fox's diagnosis. With the exception of Kidson's sloppy chemistry, there is still no clear answer for what causes this awful condition.
Notes:
(1) Although there is no strict definition of a designer drug, it usually refers to a new drug that is derived by synthetic modification of a psychoactive legal, approved drug.
(2) Neither has anyone else. There are still no good alternatives to opioids for moderate-to-severe pain.
(3) Isomers are chemicals that have the same molecular formula, but different structures and (usually) different properties.
(4) Schedule I drugs are not approved for use in people and have a high addiction potential.
(5) China White is one of the street names for fentanyl, the drug that is now killing opioid addicts in droves.
(6) Almost anyone looking at the chemical structures of MPP+ and the herbicide paraquat (below) would conclude that they would act in a similar manner. That is how similar their structures are. But they do not. Paraquat's primary toxicity is in the lungs. This is almost certainly due to the fact that the herbicide does not have properties that would allow it to cross the blood-brain barrier. Neither does MPP+, but keep in mind that MPP+ enters the brain as its precursor MPTP, which has just the right properties to do so.
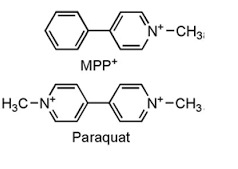
The chemical structures of MPP+ and the herbicide Paraquat—an eerie resemblance. Source: Science and Education Publishing
(7) Kidston poisoned himself in 1976. Fox was diagnosed in 1991.
(8) No matter what, if any, toxic chemical Fox was exposed to, the chances of it being a toxic batch of a Demerol analog are essentially nil. Kidston's impure drug was a one-in-a-million, freaky incident.




