There's a disturbing item in the news from Japan where at least five people have died (more likely to follow) of kidney failure after consuming to Kobayashi Pharmaceuticals Benikoji Choleste, a dietary supplement used to lower cholesterol. Although I have written numerous times about the fatal flaws of the U.S. supplement industry I'll refer you to my colleague Dr. Henry Miller's scathing 2023 article titled Death By Dietary Supplement.
Many herbal products are complex, highly variable, and impure. Not unlike the nineteenth-century snake-oil preparations that were dangerous but had little (if any) efficacy, many are toxic, carcinogenic, or otherwise unsafe. Known side effects include blood-clotting abnormalities, hypertension, deadly allergic reactions, irregular heart rhythms, kidney and liver failure, exacerbation of autoimmune diseases, and interference with conventional prescription drugs.
Dr. Henry Miller, ACSH site, 2023
What happened?
The case in Japan involves the same nonsensical "logic" used by supplement companies to circumvent all kinds of regulations and laws that govern FDA-approved drugs in the U.S. The deception is evident; even a brief look at news coverage of this event speaks volumes.
"Billed as a natural means of lowering cholesterol, the products recalled by Kobayashi Pharmaceutical Co. contain benikoji, an ingredient derived from a species of mold." (Beni koji, an alternative spelling, is also referred to as red yeast rice.)
There is more here than meets the eye, which is almost always the case in the dietary supplement world. Let's take a look.
The first statin drug – lovastatin (aka Mevinolin, Mevacor) – was isolated in 1978 from a fungal culture of Aspergillus terreus, which is commonly found in soil. It would become the first of a new class of cholesterol biosynthesis inhibitors, which acted by inhibiting a liver enzyme called HMG-CoA reductase. Blocking this enzyme reduces the amount of cholesterol made by the liver (1).
Lovastatin, the prescription drug, is no longer sold, having been replaced by more effective drugs like Lipitor and Crestor. But that's wrong. Lovastatin is still sold. Confused? Maybe a few questions will clear things up.
- Why is Benikoji Choleste Help marketed?
The supplement is sold to lower cholesterol – naturally. Assuming it does anything at all, it does so because it contains some lovastatin.

Amazon sells a number of different brands of red rice yeast.
2. Doesn't this make it a drug?
Yes, anything that treats or modifies a condition or disease is a drug. But Beni Koji sellers, just like other manufacturers and sellers of supplements, made use of common tricks to get around this problem.
First, red yeast rice (RYR) naturally contains lovastatin, a drug – a fungal metabolite.
Second, it is legal to sell RYR, which contains a prescription drug, as a food product according to our insane supplement laws. But if the manufacturers added a little more lovastatin then it becomes a drug and cannot be sold without a prescription (assuming that it was still available). Make sense? I didn't think so.
3. Doesn't the label claim that the stuff will lower cholesterol?
Yes. No. This is where logic, such as it is, breaks down.
Note that the label cleverly says that the product "supports" healthy lipid levels, which in the supplement world means "we're not really claiming anything, wink, wink," which will be interpreted by roughly 100% of the world's population as "This s##t will lower my cholesterol."
4. Isn't it a little odd that a non-drug is being made by a company called Kobayashi Pharmaceutical?
No stranger than anything else in this unfortunate chapter.
This is the sleaze factor in the supplement industry. Legal doublespeak allows supplement companies to get away with this obvious nonsense.
Why does it matter?
It matters plenty. Since supplements are classified as foods they are immune from content and purity standards that govern drugs. Which is exactly what happened. When RYR ferments it forms at least 75 organic compounds, including citrinin, a potent kidney toxin, and, according to Kyoto News, puberulic acid
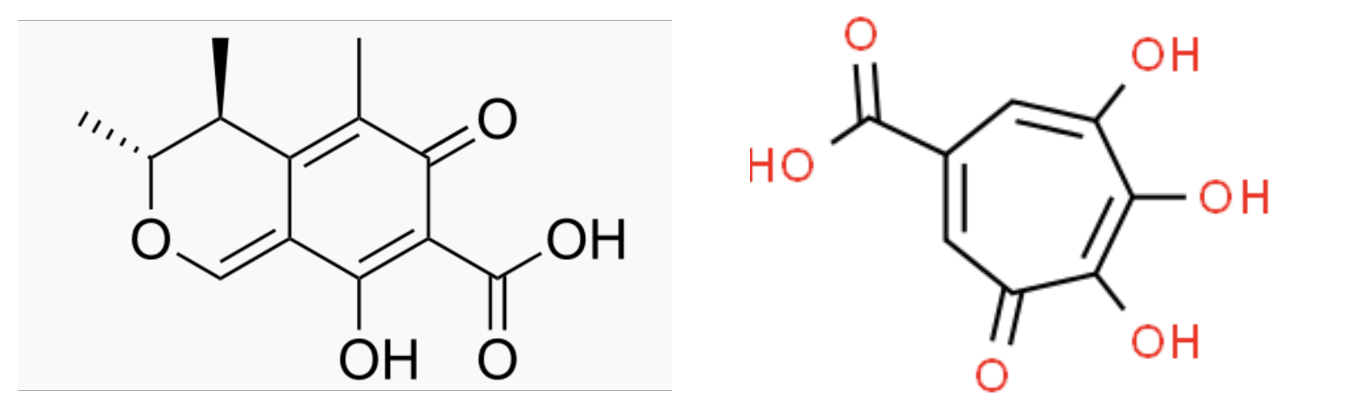
The chemical structures of kidney toxins citrinin and puberic acid.
The most likely explanation, for now, is that a bad batch(es) of the yeast contained enough citrinin to start killing people but it was never detected because ...
... according to Dr. Pieter Cohen, an associate professor at Harvard Medical School and long-time supplement critic: [my emphasis in bold]
The FDA is responsible for regulating dietary supplements. We might think of them because they're health products as being a subcategory of medication. But in fact, the FDA regulates them as a subcategory of food. This has huge consequences for the whole category of dietary supplements, from vitamins, minerals, probiotics and all sorts of new ingredients. And what it means is that the manufacturer can introduce anything into the market that they believe is safe. And the FDA's job is to identify the products that are causing harm after they've been on the market and remove them from store shelves.
Pieter Cohen. M.D., AMA's Moving Medicine
See any problem with this?
I thought so.
NOTES:
(1) Your body produces 80% of your total cholesterol. This is why diets aren't great for lowering high lipid counts.
(2) Speaking of sleaze, the FDA tried for some time to get RYR products banned on the grounds that they contained a drug. The agency succeeded but a court in UTAH threw out the decision. Why is UTAH capitalized? Funny you should ask. Utah is the home of many supplement companies, which or may not (hah!) be the reason that Sen. Orin Hatch (R, Utah) was influential in getting the insane DSHEA of 1994 rammed through Congress. Coincidence? Hard to say. But in 2001 a federal court overturned the Utah decision. Here's the court case.