What happens when regulatory ambiguity displaces sound scientific guidance, deterring the legislative intent of Congress?
biosimilars
Monsanto, perhaps to rebrand itself, and certainly to remain a leader in agricultural biotech has helped establish and fund Pairwise Plants a start-up using CRISPR-Cas9 technologies to modify seeds.
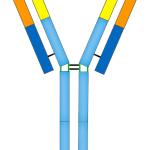
Humira, Enbrel, you see their ads regularly; they are part of a class of medications called biologics. Biologics provide relief for about 2% of America’s population at the cost of about 40% of the money we spend on pharmaceuticals.
If you follow health news the way American Council on Science and Health does, you have been treated to grandiose claims that
A big story today is that the FDA, for the first time, has ap