Monsanto, perhaps to rebrand itself, and certainly to remain a leader in agricultural biotech has helped establish and fund Pairwise Plants a start-up using CRISPR-Cas9 technologies to modify seeds. They have given the new company money and their vice-president of global biotechnology to head the start-up, so this is a strategic business move not simply hedging their bets. This is important because CRISPR-Cas9 may genetically modify a crop, but it doesn’t necessarily result in a genetically modified organism, the dreaded GMO. To understand how that can be, we need to understand both genetic modification and federal regulations.
Mutagenesis
Mutagenesis broadly refers to creating a change in DNA (a mutation), and until the advent of agriculture, about 10,000 years ago, it was a spontaneous event - the driving force of evolution. Humans have been genetically modifying crops since then, beginning with the domestication of wild grasses like wheat and rice. Through cross-breeding, grafting and close observation, farmers observed trait (or phenotype) improvements that led to stronger crops.
It was only in the mid-nineteenth century that Gregor Mendel's work that described genetic inheritance in pea plants opened the doors to the led to the concept of genetic modification.
The earliest attempts at intentional mutagenesis can be traced back to shortly after World War II when atomic energy captured our interest. There were governmental studies of the effects of radiation on plants, at places like Brookhaven Laboratories on Long Island and even amateur Atomic Garden Societies obtained a radiation source to see what they could grow. [1] But radiation just resulted in more random mutations. The search for a means to change specific traits efficiently was begun.
Genetically Modified Organisms and CRISPR-Cas9
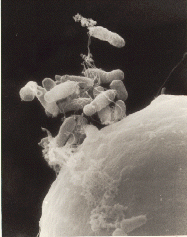 There are now a variety of ways that DNA can be altered. One of the more common methods, before the introduction of CRISPR-Cas9 techniques involved using a bacteria that causes plant tumors, Agrobacterium tumefaciens, (shown on the left) to introduce a new genetic sequence into the target plant. Through a bit of biomolecular wizardry, the genes in A. tumefaciens causing the tumor can be replaced with genes from other plants or species that we want to insert. The recombinant DNA, made up of both newly added foreign DNA as well as the plant’s normal DNA can then produce offspring. This technology has been used to create GMO versions of several crops including soybeans, corn, and rice. But the technology uses a known plant pest to insert a foreign piece of DNA somewhere into a plant’s genome. While better than random mutagenesis it is still not completely specific or efficient.
There are now a variety of ways that DNA can be altered. One of the more common methods, before the introduction of CRISPR-Cas9 techniques involved using a bacteria that causes plant tumors, Agrobacterium tumefaciens, (shown on the left) to introduce a new genetic sequence into the target plant. Through a bit of biomolecular wizardry, the genes in A. tumefaciens causing the tumor can be replaced with genes from other plants or species that we want to insert. The recombinant DNA, made up of both newly added foreign DNA as well as the plant’s normal DNA can then produce offspring. This technology has been used to create GMO versions of several crops including soybeans, corn, and rice. But the technology uses a known plant pest to insert a foreign piece of DNA somewhere into a plant’s genome. While better than random mutagenesis it is still not completely specific or efficient.
The CRISPR-Cas9 system more identifies explicitly target genes and modify them by deleting or insertion something as small as a single base pair or as large as a gene. As a result, it can change genetic behavior, turning on or off genes, even creating new traits. CRISPR-Cas9 doesn’t require foreign genes, nor does it necessarily need a bacterium to introduce the system into the target cells. It leaves no trace, and the genome that has been modified is almost identical, biosimilar is the word I would choose, to the original.
Regulation
Scientific concerns about the impact of genetic modification, especially recombinant DNA have existed since the introduction of these techniques and in 1986 regulatory oversight was apportioned to the EPA, USDA, and FDA. The EPA is involved in genetic changes that impact pesticide use, their chemical mandate. The USDA considers whether genetic changes result in “plant pest” concerns – weeds overtaking healthy crops. Until their safety concerns are met, and that can take years, these plants are not allowed out of the laboratory. The FDA’s program is voluntary, all manufacturers have complied, to determine whether the modified crop is “substantially equivalent” to the non-modified version. They are concerned about allergenicity and the introduction of new toxins. In every case, how the plant was created is not at issue, it is the final product that is regulated.
The USDA has determined that CRISPR-Cas9 techniques when they do not introduce foreign DNA or use a “plant pest” bacteria for insertion do not require their oversight. In the case of the non-browning mushrooms the final product had no evidence of CRISPR-Cas9 or antibiotic markers used in the development process – it was simply the same white button mushroom, Agaricus bisporus, with a small deletion of the enzyme responsible for browning. A CRISPR-Cas9 genetically modified or engineered product that contains traces of A. tumefaciens plasmid or a gene from another species (transgenic) would trigger USDA review and ultimately classification as a GMO.
The response to these regulatory decisions by the anti-GMO forces has been to cry foul. For them, any alteration of DNA is genetic manipulation – it is all about the process. But if that were the case, then crossbreeding and grafting techniques we have used for hundreds of years would also be of concern. Why is poorly directed or random mutagenesis any safer than newer technology? Aren’t they concerned about how the product impacts our environment and health, shouldn’t the end product be the real concern?
Global scientific thought believes that a crop’s safety is based on the end product not the mechanism of mutagenesis. [2] Even the World Health Organization, the people that bring you IARC’s hot water is a carcinogen, believe that “establishment of substantial equivalence provides sufficient assurance that the foods and food components obtained from a plant-derived by modern technology are comparable, regarding safety, to their conventional counterparts.”
[1] For a wonderful podcast in general and an excellent presentation on Britain’s Atomic Garden Society, you can click here.
[2] Besides our scientists this includes the UK Biotechnology and Biological Sciences Research Council, The German Federal Office for Consumer Protection and Food Safety, the French High Council for Biotechnology, and the UK Advisory Committee on Releases to the Environment