Just three business days after receiving the stamp of approval from federal regulators, a more effective shingles vaccine will receive the attention of the Centers for Disease Control on Wednesday, when a vote is expected about recommending its use to the general public.
The CDC's Advisory Committee on Immunization Practices will discuss how to add Shingrix, which was approved late Friday by the Food and Drug Administration, to its Adult Immunization Schedule. The vaccine will find its place among other recommended vaccines and shots, with this particular medicine slated for adults 50 and older, according to its maker GlaxoSmithKline. The CDC, however, says the vaccine is best suited for adults 60 and older because its potency can weaken over time.
Wednesday's committee meeting also spotlights the schedule itself, providing an opportunity to raise it especially given that many middle-aged adults are likely unaware even of its existence. So here it is, and here's the link to the CDC's website if you'd like more detail on each of the vaccines listed on the left.
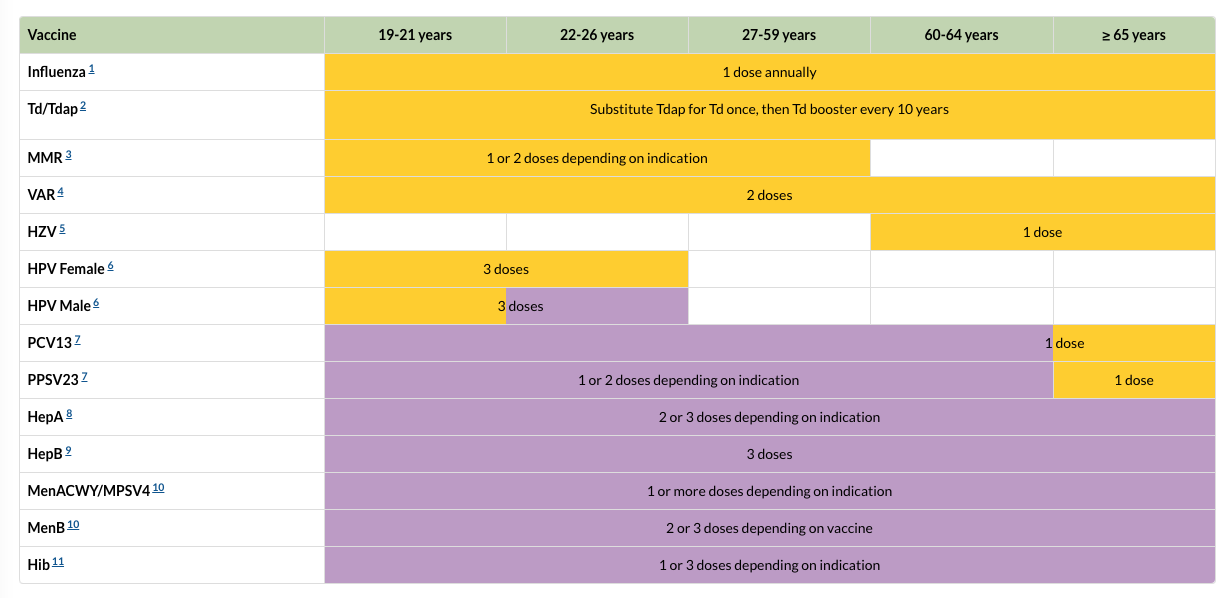

VACCINES, as abbreviated above (left):
- 1. Influenza
- 2. Tetanus, diphtheria, and acellular pertussis
- 3. Measles, mumps, and rubella
- 4. Varicella vaccination
- 5. Herpes zoster (for shingles)
- 6. Human papillomavirus
- 7. Pneumococcal
- 8. Hepatitis A
- 9. Hepatitis B
- 10. Meningococcal
- 11. Haemophilus influenzae type b
Shingrix improves upon Zostavax, the only other FDA-approved shingles vaccine, which drugmaker Merck brought to market in 2006. Shingrix is 90 percent effective while Zostavax was shown to work about 50 percent of the time. As we wrote last month about the vaccine's benefits, Shingrix sailed through the FDA's advisory panel with a unanimous vote in favor of approval.
What's more, Shingrix is engineered to reduce nerve pain by 90 percent, and as most people are aware that's critically important because shingles can be extremely painful, and it can remain present for months, or even years.
While GSK's current production of the vaccine will make it available soon, it's now up to the ACIP, the CDC's panel, to formally recommend how often it should be administered, and to what age group. In trials, Shingrix was shown to be effective when administered in two doses, the second eight weeks after the first.
Shingrix was also approved in Canada last week.



