A little luck and a clever idea can go a long way in finding treatments for unmet medical needs. One way to do this is by making use of drug repurposing - the process of discovering "new" drugs from "old" ones. In other words, starting with approved drugs and finding another disease for them instead of the other ways around (standard drug discovery). This approach has been used to identify and already approved hepatitis C drug that also has the potential to treat two other viral infections. One of these makes sense. The other - not so much.
The advantages are enormous. It takes 10+ years and billions of dollars to get a drug to the pharmacy. Discovery takes roughly one-third of this time and development (safety, efficacy...) takes up most of the rest. By using known drugs, which have already safety data at multiple doses, both of these steps are eliminated. Many repurposed drugs were discovered by accident, which will only work so well and so often, although technology is changing this.
Here are some examples. You probably are familiar with some of them.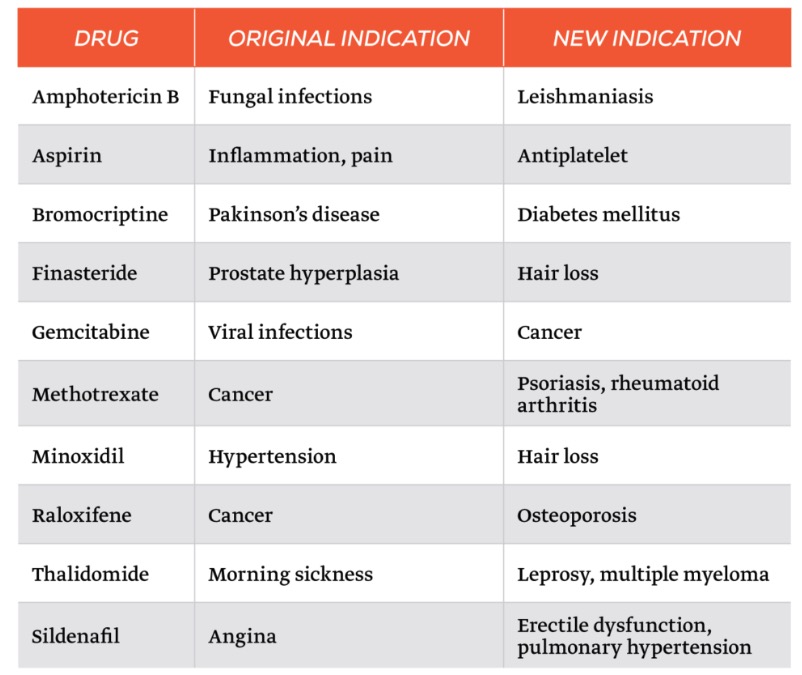
Examples of repurposed drugs. Source: Geneva Network
The discovery that sofosbuvir (Sovaldi - the "thousand-dollar pill,") the first direct-acting antiviral drug that cured (1) hepatitis C (See Sovaldi $84,000 And Still Quite A Bargain) is potentially useful against two other very bad viral infections - Yellow Fever and Chikungunya - is both expected and unexpected:
"Drug development is extremely costly and time-consuming. The process takes 12 years on average between the start of research and bringing the drug to market. The cost is on the order of US$1.5 billion or more...sofosbuvir has been fully approved for human use. This means it can be readied for use against chikungunya in one to three years, for a much lower cost, estimated at approximately US$500,000.
Lucio Freitas-Junior in F1000 Research
Here's how Lucio H. Freitas-Junior and colleagues at the University of Sao Paulo in Brazil pulled it off. The group used high throughput screening (1), a method that is a staple of drug discovery research, and tested a panel of 1,280 chemical compounds (including approved drugs) looking for anything that would inhibit the replication of Chikungunya or Yellow Fever. Out popped sofosbuvir. This result is both expected and unexpected.
Expected
Hepatitis C virus (HCV) is a member of the Flaviviridae family of viruses. So is Yellow Fever. So it is not unreasonable to conclude that one drug might be effective against two different viruses from the same family. Nor is it a given. For example, although acyclovir (2) is effective against oral and genital herpes simplex, shingles (varicella-zoster), and chicken pox, it does not work well against cytomegalovirus infection. All four are members of the herpesvirus family. So just because HCV is related to Yellow Fever this does not mean that both will be susceptible to the same drugs.
Unexpected
It is less clear why sofosbuvir should have antiviral properties against Chikungunya. Chikungunya is spread by mosquitos; hepatitis C is not. More relevant is that Chikungunya, which is nothing you want to get, is a member of the Togaviridae family of viruses, one of which, the rubella virus, causes German Measles. Unlike antibiotics, which hit many unrelated bugs, antiviral drugs are much more selective. So it would be unusual to find a drug that worked against Chikungunya and Yellow Fever.
Why it worked anyhow
The key word here is "worked." More specifically, how well did sofosbuvir inhibit Chikungunya? Unfortunately, the answer is not that well.
"Among tested compounds, sofosbuvir, an anti-hepatitis C virus drug, exhibited good selectivity against CHIKV with an EC50 of 11 µM, suggesting it is a promising candidate for repurposing."
If you don't know virology those numbers won't mean anything, but they are easily explained. Perhaps even enough so that the Environmental Working Group might understand.
Nah. Not that easy.
The term EC50 is an abbreviation for the effective concentration at which the replication is reduced by 50%. The EC50 is a measure of how potent the inhibitor (sofosbuvir) is against Chikungunya. The answer is "not very." It takes a concentration of 11 micromolar of the drug, which is not very potent as antiviral drugs go, to get the 50% reduction in replication. But for HCV, EC50 numbers range from 0.014 to 0.11 micromolar, depending on the strain of the virus. This means that sofosbuvir is about 100-800 times less effective in inhibiting Chikungunya than it is in inhibiting HCV. Although this does not mean that it would take a dose of 100-800 fold more to treat Chikungunya it is virtually certain that more drug would be required, and probably much more. The daily dose of sofosbuvir to cure HCV infection is 400 mg, and at that dose side effects include headache, fatigue, nausea, and vomiting (3). I would not want to be the one trying 40,000 (40 grams) or 320,000 mg (320 grams) of the stuff. These doses, which are completely unrealistic, would almost certainly be fatal.
So, even when you start with a drug that is approved for one disease, it is still plenty challenging to make it work for another. But there is nothing simple about drug discovery. It's the hardest job in the world.
NOTES:
(1) This isn't really high throughput screening. HTS involves the testing of hundreds of thousands (or millions) of distinct chemical compounds against the target. Mediocre throughput screening just didn't sound so great.
(2) Valacyclovir (Valtrex) is preferred for herpes. It is a pro-drug of acyclovir, meaning that it is inactive but gets metabolized in the blood to give acyclovir.
(3) This is not a fair comparison because Sovaldi is only approved for use with ribavirin or interferon, which have significant side effects of their own.