The mad rush to purge this country of demonic prescription analgesic drugs (mainly Vicodin and Percocet) has pain patients and their doctors in an unprecedented dilemma. The tapering or complete discontinuation of painkilling drugs that pain patients have been using successfully for years, even decades, and the reluctance of physicians to write new prescriptions has left a vacuum, which was filled by drugs or therapies that were unapproved, worthless, or both.
There is another term for this: Human Experimentation. Last I looked this was frowned upon, except in Nazi Germany. One would hope that humanity had progressed to the point where this sort of thing might be considered unacceptable. And it was, at least until 2016, when the CDC, with the "help" of Physicians Responsible for Opioid Prohibition (PROP) (1) decided that it was time to take chronic pain patients off opioid drugs without having anything resembling a decent plan for what to do with them. This, by necessity, forced patients and their physicians to try other untested drugs and modalities (2) without having evidence that either would work. Nice work, fellas.
Some of the alternatives that were shoved down patients' throats included Tylenol (useless, dangerous), NSAIDS (stomach and kidney killers), antidepressants (please) and most importantly Neurontin (generic name gabapentin and to a lesser extent Lyrica (pregabalin). Lyrica and Neurontin are so important that all two of them were recognized as a new class of drugs with a pretentious name - gabapentanoids (3).
Since Neurontin is now being handed out like Butterfingers at Halloween it is fair to ask whether there is evidence that it works. Fortunately, Drs. Christopher Goodman and Allan S. Brett ask just this in "A Clinical Overview of Off-label Use of Gabapentinoid Drugs," which was published as JAMA Network Special Communication. Here are the highlights.
In 1993 Pfizer received FDA approval for Neurontin for the treatment of seizures. Shortly thereafter Pfizer began to push the drug's off label indication for pain. In 2002 Neurontin was approved for pain, but only for postherpetic neuralgia. In 2004 Pfizer/Warner-Lambert pled guilty to improperly marketing the drug for off-label uses, coughed up 430 million of its favorite dollars and faced a class action lawsuit, all of which is documented by Landefeld and Steinman in 2009 (4).
Let's look at some of the findings from Goodman and Brett.
- The authors searched the PubMed database in September 2018, using the search terms "pain and gabapentin" (5) They excluded studies involving cancer pain and headaches.
- The search turned up 54 studies, 34 of which were randomized controlled trials (very good) and 18 also included placebo in the study (even better). A total of 22 studies encompassing 13 painful conditions was selected. These are presented below. Prepare to be unimpressed.
TWENTY-TWO STUDIES RANDOMIZED CLINICAL TRIALS COMPARING NEURONTIN AND PLACEBO FOR 13 PAINFUL CONDITIONS
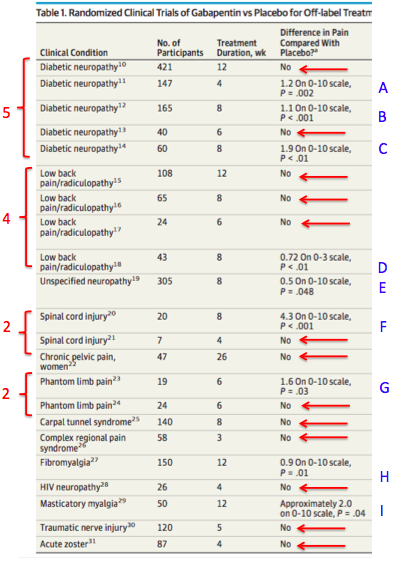
Table 1. Twenty-two randomized controlled trials examining the efficacy of Neurontin in treated 11 different painful conditions. Adapted from "A Clinical Overview of Off-label Use of Gabapentinoid Drugs, Christopher W. Goodman, MD; Allan S. Brett, MD JAMA Intern Med. 2019;179(5):695-701. doi:10.1001/jamainternmed.2019.0086
SUMMARY OF TABLE 1
- Diabetic neuropathy (5 studies) - Studies A, B (blue letters) showed a statistically significant but very small difference between Neurontin and placebo for treating diabetic neuropathic pain. Study C showed a larger difference between the two.
- Lower back pain with radiculopathy - Of the four studies, 3 showed no difference between Neurontin and placebo. Study D showed a significant difference between the two.
- Unspecified neuropathy. One study (E) showed a statistically significant but minuscule difference between Neurontin and placebo. I don't care what the statistics say. A difference in pain score of 0.5 on a scale of 0-10 means nothing, especially since pain is subjective.
- Spinal cord injury pain. A small study (F) showed a significant difference between Neurontin and placebo. A smaller study showed no difference.
- Chronic pelvic pain in women. No difference.
- Phantom limb pain. One study (G) showed a small effect. Another showed none.
- Carpal tunnel syndrome. No difference.
- Complex regional pain syndrome (CRPS). No difference.
- Fibromyalgia. Statistically significant but minuscule difference between Neurontin and placebo.
- HIV neuropathy. No difference.
- Masticatory Myalgia (facial pain from muscle spasms). A small but significant difference between Neurontin and placebo
- Traumatic nerve injury. No difference.
- Acute herpes zoster (shingles) pain. No difference.
IS THIS STUFF GOOD FOR ANYTHING?
Perhaps I missed it, but there ain't much in here that even suggests that Neurontin has any utility whatsoever as a pain medication. In 59% (13/22) of the randomized controlled trials, it did not perform any better than placebo. In 18% (4/22, trials A, B, E, and H)) the difference between Neurontin and placebo was statistically significant but very small. Let's toss them in with the first group and we have 77% of the trials showing little or no utility of Neurontin in treating pain. Of the remaining five trials - those that seemed to indicate some effect - three (14%, C, G, and I) had only a modest effect (pain reduction score of ~2 on a scale of 1-10).
That leaves two trials where a robust effect was seen. In trial D (lower back pain) a pain reduction score of 0.72 on a scale of 1-3 seems to be significant, but keep in mind that in the three other trials (above this one in Table 1) for lower back pain there was no difference between Neurontin and placebo. What does this mean? Who knows?
The only study (F) where Neurontin shows significant efficacy (a 4.3 reduction in pain score on a 0-10 scale) consists of 20 patients.
So it cannot be surprising that the authors conclude:
Clinicians who prescribe gabapentinoids off-label for pain should be aware of the limited evidence and should acknowledge to patients that potential benefits are uncertain for most off-label uses.
C. Goodman and Allan S. Brett, MDs
So, it's certainly fair to say that Neurontin is just about useless as a pain medication, at least most of the time. Yet it is being used like crazy, and there can be no one more responsible for its (mis)use than Dr. Andrew Kolodny, the godfather of the anti-opioid crusade. Which made me wonder what he had to say about the drug.
Prepare yourself...
"[Because of the abuse potential, Neurontin] should probably be scheduled as a controlled drug. Then people can’t doctor-shop for it. That’s where drugs with abuse potential belong,”
Andrew Kolodny, MedShadow April, 2018
That might be the funniest thing I've ever heard.
NOTES:
(1) A few months ago, in the interest of accuracy, I renamed Physicians for Responsible Opioid Prescribing (PROP) Physicians Responsible for Opioid Prohibition (PROP). No one has complained so far, so I'll assume that the name is now official.
(2) Don't you just want to smack someone who uses the word modalities? Pretentious much?
(3) Gabapentanoids???? Are you kidding me? There are TWO of them - Neurontin and Lyrica. They don't deserve a classification, especially one ending in "oid." Likewise, the assignment of the terms "opioid" and "opiate" is a false distinction, because it is based on the source of the drug, not the structure. This is a chemical no-no; the source of a chemical or drug is irrelevant. It is only the chemical structure that matters. Many think that "opioid" was invented to confuse people and cloud the issue.
(4) See: Landefeld CS, Steinman MA. The Neurontin legacy: marketing through misinformation and manipulation. N Engl J Med. 2009;360(2):103-106. doi:10.1056/NEJMp0808659
(5) Goodman and Brett also did the same searches for pregabalin (Lyrica). I have omitted these results to focus only upon Neurontin.



