
Did you ever take a sip of a Diet Coke and have it taste like death (1)? Not at all sweet. Kind of sour-metallic-motor oil-rotting corpse-tasting? Well, since artificially sweetened drinks are already known to be bad for you, (2) you can be sure that whatever's in that can only be worse, right? But it doesn't really matter because instead of swallowing it, you just spewed it all over the guy across the table from you.
The good news is that nothing harmful went into your mouth in the first place, even though it tasted awful. We'll look at that below. The bad news is that the guy you spewed on is a former Mixed Martial Arts champion who was wearing a $4,000 Armani suit while on his way to a court-mandated anger management session at the time of the spewage. He was not pleased.
WHAT CAUSED THE SPEWAGE?
Perhaps you can take some small comfort, as you are being hooked up to assorted machines in your favorite trauma unit, is that what got you into this unfortunate situation was a chemical reaction invented by the Babylonians around 2800 BC – the hydrolysis of an ester. Back then, the ester used was olive oil and the product they made was the first soap. It's essentially the same reaction that made the Diet Coke taste nasty (3) (Figure 1):
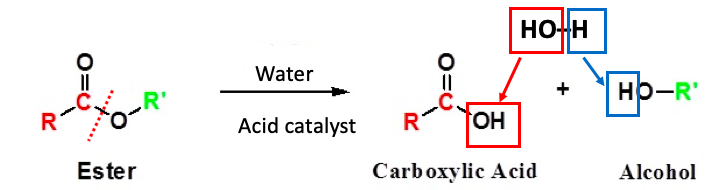
Figure 1. The acid-catalyzed hydrolysis of an ester. (Left) An ester is "made up" of two pieces – a carboxylic acid and an alcohol – minus a molecule of water. The red hatch line shows an ester bond, which connects the carboxylic acid and the alcohol. It is this same bond that breaks during the hydrolysis (breakdown due to reaction with water) reaction. (Right) During the hydrolysis reaction the hydroxyl (OH) group that is incorporated into the carboxylic acid (red square) comes from the hydroxyl in water. The remaining hydrogen atom in water (blue square) ends up attached to the alcohol that is formed.
WHY ASPARTAME?
Figure 2 shows the chemical structures of some common artificial sweeteners. Note that aspartame contains an ester group (red circle), which renders it susceptible to acid-catalyzed hydrolysis. None of the other sweeteners conatins an ester, so they remain stable under the conditions that aspartame breaks down. 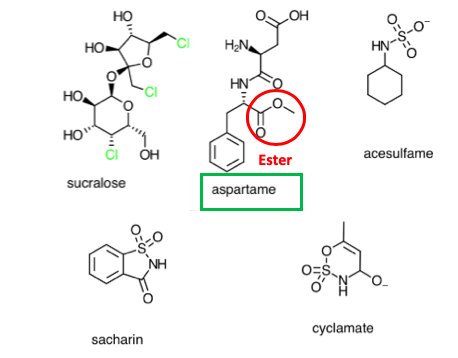
Figure 2. Structures of some common artificial sweeteners. Source: Stack Exchange.
The rate of the hydrolysis reaction of aspartame (found in Nutra Sweet, Equal, others) depends on the pH of the soda solution (Table 1). At pH 3.25, which is virtually identical to that of Diet Coke, about half of the aspartame will be gone after 5 months in a warm room.
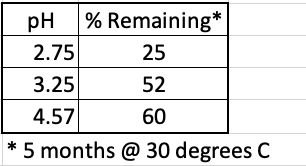
Table 1. Stability of aspartame at different pH values after five months at 30º C (86º F). Source: International Journal of Food Properties
The temperature at which the drink is stored also affects the stability of the aspartame (Table 2). Hot warehouses are bad.
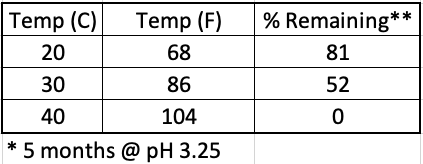
Table 2. The impact of storage temperature on aspartame stability at pH 3.25. In a cool room, most of the aspartame remains after five months. In a hot room, all of it is gone.
WHAT HAPPENS TO THE ASPARTAME, AND WHY DOES THE SODA TASTE ICKY?
The reasons why I wrote that virtually unreadable section on ester hydrolysis were 1) to torture you, 2) to show why Diet Coke goes bad in old or poorly stored cans. Here's the reaction:
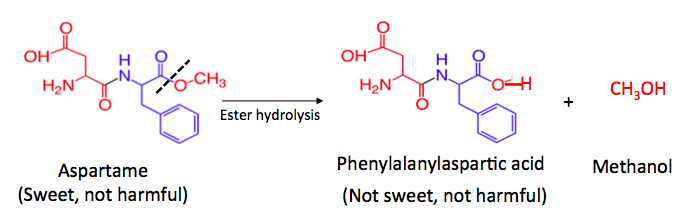
When the ester group in aspartame hydrolyzes, just like the generic reaction at the beginning of the article, the products are a carboxylic acid and an alcohol. In this case, the carboxylic acid is called phenylalanylaspartic acid and the alcohol is methanol (4). But, phenylalanylaspartic acid, which no longer contains the ester, is not sweet. When a Diet Coke sits around too long it is the absence of sweetness that causes the unpleasant taste, not some hideous poison.
Phenylalanylaspartic acid breaks down further, either in the can or in your stomach. Proteins and peptides (5) are broken down by water or a group of enzymes called peptidases to give back the individual amino acids that were put together to make the peptide or protein. Phenylalanylaspartic acid does just this. In a sense, phenylalanylaspartic acid can be considered a "food," just like dietary protein; it breaks down to form aspartic acid, and phenylalanine– two of the 20 amino acids that make up proteins.

All dietary proteins contain aspartic acid and phenylalanine, so whether you consume aspartame, phenylalanylaspartic acid, or a chicken leg, your body will digest them and you will be getting some phenylalanine and aspartic acid. This is why neither the sweet nor the sour diet soda is harmful; your body just sees them as food.
Try telling that to the Mixed Martial Arts guy who just put you in a full-body cast.
NOTE:
(1) I arbitrarily chose Diet Coke because a) I like it b) it contains aspartame.
(2) That's a lie. Artificial sweeteners are harmless, no matter what the Internet says.
(3) Esters hydrolyze much faster under basic conditions. The Babylonians heated caustic soda (sodium hydroxide, lye) with olive oil (animal fat works too) to make soap. The same method is used today. The process is called saponification.
(4) Small amounts of other byproducts form when aspartame is hydrolyzed. These are beyond the scope of this article.
(5) Peptides are chains containing 2-50 amino acids hooked together by amide bonds. When larger numbers of amino acids are present then it is called a protein. A bit arbitrary.



