Even if an effective vaccine for the Wuhan coronavirus is discovered, which is by no means a certainty, it will be at least a year (more likely two) before you'll be rolling up your sleeve. And it is by no means certain that an effective vaccine will be found at all. Despite years, in some cases decades, of research, there are still no vaccines for a number of important infections, such as HIV/AIDS, hepatitis C, norovirus, Lyme and herpes simplex.
Although it is better to prevent an infection than treat one, sometimes drugs can be so effective in treating infections that the distinction becomes nebulous. For example, HIV/AIDS was an automatic death sentence prior to 1995, but it is now so well-controlled by very potent drugs that HIV+ people lead lives that aren't very different from those without the infection. And because of a massive 25-year drug discovery campaign, hepatitis C (HCV), which has infected more than 3 million Americans, can now be cured in 95+% of the time.
We should all cross our fingers and hope that remdesivir, Gilead Science's experimental antiviral drug which is now being evaluated in multiple clinical trials in China, will work even partially as well as the AIDS and HCV drugs. If so, Gilead, the world's premier antiviral pharmaceutical company, will have pulled off another miracle.
And right now we need one.
WILL REMDESIVIR WORK AS WELL AS OTHER ANTIVIRAL DRUGS?
Quite possibly. Remdesivir lies within the range of potency of drugs used for other viral infections (see Figure 1) in cell-based drugs (in vitro) assays (tests) (1,2).
Cell-based assays – mini-experiments run in tiny wells – measure how well a potential drug will inhibit (or promote) a particular process that is relevant to the disease or infection in question. For example, in cancer research, the goal of a project may be stopping a particular type of cell from dividing. This can be indirectly measured in vitro (out of the body) by exposing isolated cells to different concentrations of a potential drug and determining whether the division is inhibited.
Likewise, an antiviral drug must stop viral replication in vitro or there is no hope that it will do so within a human body (3). This is the beauty of automated cell-based assays. They can quickly test hundreds of thousands, even millions, of chemical compounds to see if any of them perform the desired result, in this case inhibiting the replication of the coronavirus, and if so, how well. But cell-based assays are only part of the process in drug discovery.
Three critical factors (there are others) that determine whether an antiviral drug that performs well in vitro will be effective in humans include:
- Potency – How much drug is required to inhibit the replication of the virus (the less, the better)?
- Selectivity/Toxicity – Is there a sufficient difference between the concentration of the drug needed to inhibit replication vs. the concentration that is toxic to cells?
- Bioavailability/Metabolism – Will the drug be sufficiently absorbed into the bloodstream and be metabolically stable enough to reach its designated target in a sufficient quantity?
Let's take a look at all three.
1. POTENCY
It is intuitively obvious (and often true) that a drug which requires only a small dose to inhibit viral replication (more potent) is going to be superior to another drug that requires a large dose (less potent). The in vitro potency of a drug is defined as the concentration required to inhibit 50% of the process being measured, in this case, coronavirus replication. This parameter is called the EC50, which stands for Effective Concentration 50%. The lower the EC50, the more potent the drug. EC50 concentrations are given in molarity – one method of expressing concentration. For the purposes of this article we need not define molarity, but rather, just compare the molarity/concentration of different drugs. Figure 1 shows the relative potency of remdesivir and three known antiviral drugs (3). Its potency lies within the range of the three.
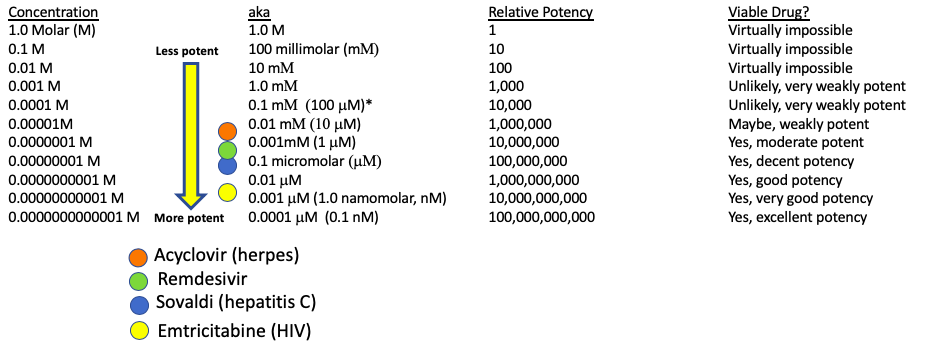
Figure 1. The potency of four antiviral drugs in cell cultures. Emtricitabine is about 100-times more potent, and Sovaldi. which is about 10-times more potent than remdesivir. Acyclovir is roughly 10-times less potent than remdesivir.
2. SELECTIVITY AND TOXICITY
But, the potency of a potential drug is only one parameter in a cell-based assay that must be considered. Cellular toxicity can be equally as important. This is because viruses will only replicate in healthy host cells, so a compound that kills the host may also appear to be an antiviral, but it is just a cellular toxin. Toxicity is measure by CC50 (the Cytotoxic Concentration that kills 50% of the cells). The ratio of potency to cellular toxicity is called the selectivity index (SI), another key parameter in assessing whether a chemical compound can make a useful antiviral drug. The higher the SI the more selective the drug. Selective drugs tend to be less toxic, although there are other factors that determine overall toxicity.
Data comparing the anti-coronavirus potential of remdesivir and six other known antiviral drugs was reported in a letter to the editor in the February issue of the journal Nature (Figure 2). It should be obvious why remdesivir was selected to be tested in clinical trials.
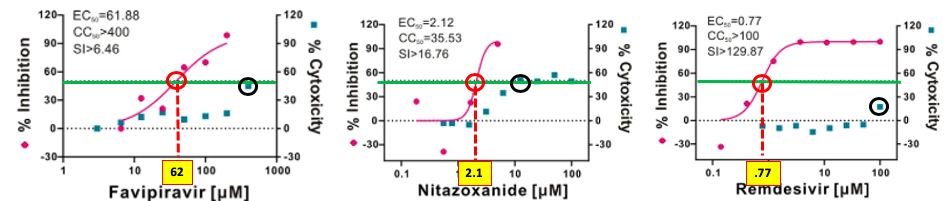
Figure 2. Dose-response curves of three drugs.The potency, toxicity, and selectivity of three potential coronavirus drugs tested by Wang et. al. The green line shows 50% inhibition. The place where the pink curve (potency) meets the green line (red circle) is the EC50 (yellow square). The blue squares are data points of cellular toxicity at different concentrations of the drug.
Figure 2 tells us that favipiravir has an EC50 of 62 micromolar (a high number for a drug), and a CC50 of greater than 400 micromolar which gives it an SI of >6.46 (400 divided by 62). Favipiravir is unlikely to be a successful coronavirus drug. Nitazoxanide is 30-times more potent than favipiravir (EC50 = 2.1 micromolar, but is considerably more toxic; its CC50 is 35.5 micromolar, giving it an SI of ~17, which isn't great. Nitazoxanide is a better candidate than favipiravir but is uninspiring because of its low potency and mediocre SI.
But remdesivir is a different story. Its EC50 (0.77 micromolar) puts it in the range of the three known antiviral drugs (Figure 1) and its toxicity is very low (CC50 is greater than 100 micromolar) making the SI more than 130 (4).
Potency: Remdesivir is a moderately potent antiviral drug. GRADE: B
Selectivity: There is no apparent toxicity. GRADE: A
3. BIOAVAILABILITY AND METABOLISM
Bioavailability is a measure of how well an orally administered drug gets into the bloodstream. If the bioavailability is 10% this means that only a small amount of the drug is found in the blood. The rest of the drug can either pass unchanged through the gastrointestinal tract and be excreted in the feces or get absorbed by the gut and be metabolized by the liver. The metabolites are then (usually) excreted in the urine.
Pills are usually preferable to injectable solutions because of the ease of storage and administration, but in a world-wide emergency, especially when so many people are critically ill, IV administration is acceptable, if not preferable. So, bioavailability is a non-issue. GRADE: N/A
The metabolism of remdesivir in rhesus monkeys was reported by Warren, et. al., at a time when the drug was being evaluated as a therapy against Ebola (it failed). But these studies show that "[remdesivir] efficiently delivers drug metabolites (5) to sanctuary sites where virus may persist." GRADE: B+
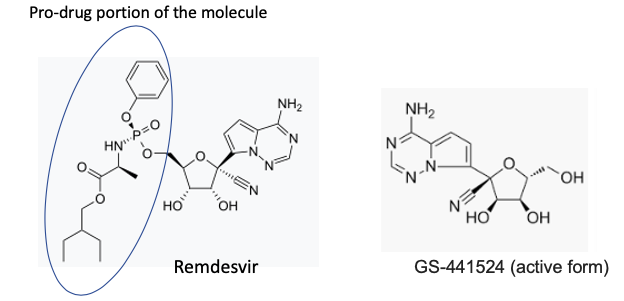
The chemical structures of remdesivir (right) and GS-441534, the active species. The pro-drug fragment is indicated by the blue oval.
OTHER INFORMATION
Remdesivir has been shown to inhibit the growth of SARS-and MERS replication in human airway epithelial cells.
The drug was given to a dying patient, who subsequently recovered, although it is impossible to tell the efficacy of the drug from a single person.
PREDICTION:
Remdesivir is a fairly potent inhibitor of coronavirus replication with no obvious toxicity. It may or may not work as a pill but this is irrelevant at this time since it will probably be given IV. In another study in rhesus monkeys, data indicated that a significant concentration of the drug will be found in the blood and that an adequate quantity of the drug should be delivered to the sites where it is needed.
Given the properties of remdesivir as well as the desperate need for a coronavirus therapy, I would estimate that the drug has at least a 50% chance of becoming the first drug to treat the virus. (5)
Let's hope I'm right.
NOTES:
(1) In vitro tests are not run in test tubes like in the movies. Very small glass wells are used, but the concept is the same.

Multiple compounds are tested in plates containing many small glass or plastic wells. Photo: Materials Today
(2) Some assays do not use cells. Instead, specific targets within the cell, for example, enzymes or other proteins are tested. These are called acellular assays and are beyond the scope of this article.
(3) The exception to this rule is pro-drugs. Pro-drugs will not have antiviral activity in cell culture because they depend upon metabolism to unlock the active drug, usually in the bloodstream.
(4) Although a high SI in a cell-based assay is important this number says little about the toxicity in an animal or human. Toxicity can show up in different types of cells, organs, etc.
(5) Remdesivir is not the only drug being evaluated for coronavirus. My colleague Alex Berezow wrote about an arthritis drug that may be useful.
Disclaimer: My IRA contains a small amount of Gilead stock. It seems to be getting smaller every day.



