Several years ago, a brand new method of genetic engineering called CRISPR was invented, and it was based on discoveries made about the rudimentary "immune system" possessed by bacteria. Essentially, bacteria have a way of "remembering" which viruses had infected them previously, and they possess a molecular system that destroys viral DNA that matches that of a prior infection.
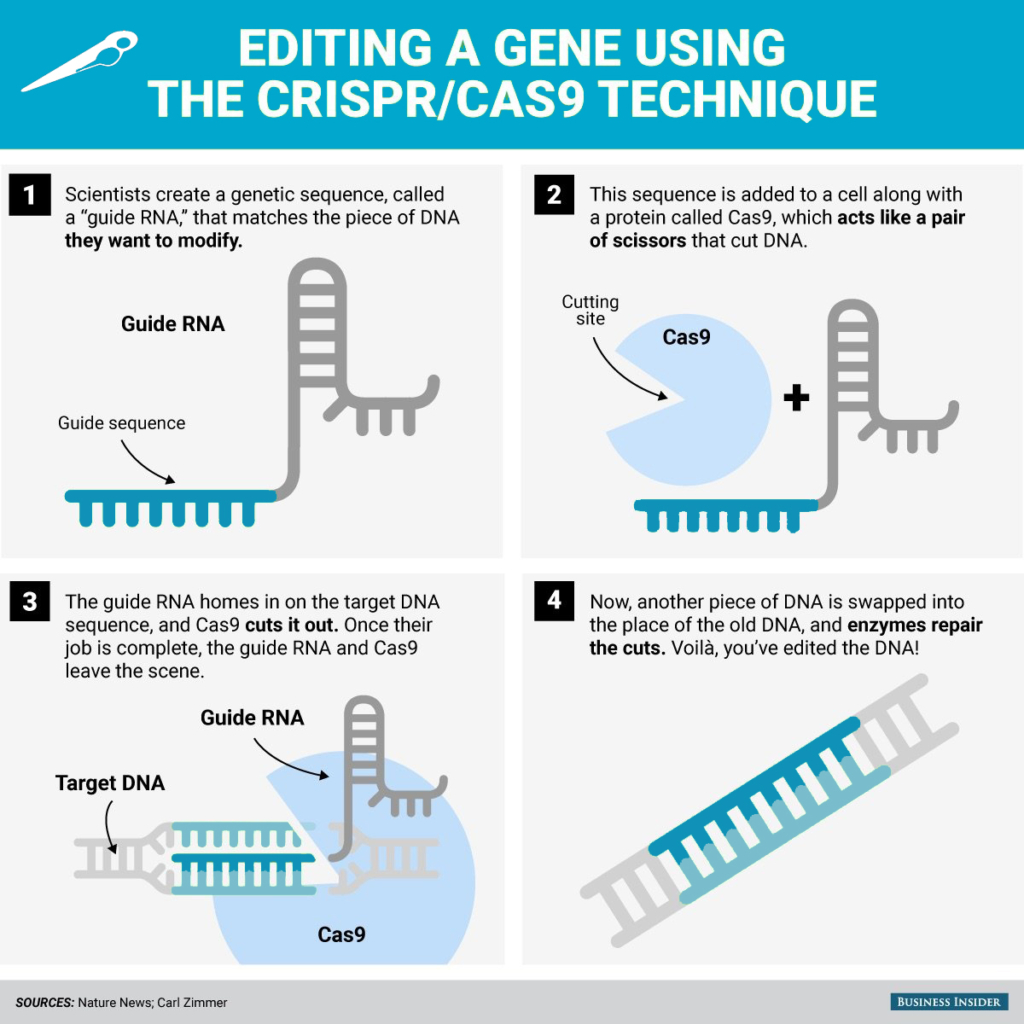
The molecular system consists of a DNA-cutting protein called Cas9. (See infographic from Business Insider below.) When equipped with a special guide RNA, Cas9 can be used to cut specific DNA sequences, for instance, a mutated gene that is causing a health problem. Because a broken DNA molecule is dangerous, the cell will attempt to repair it. If a DNA segment is snuck into the cell before the repair occurs, the cell can insert the new (and usually improved) DNA piece, providing a method to "edit" DNA.
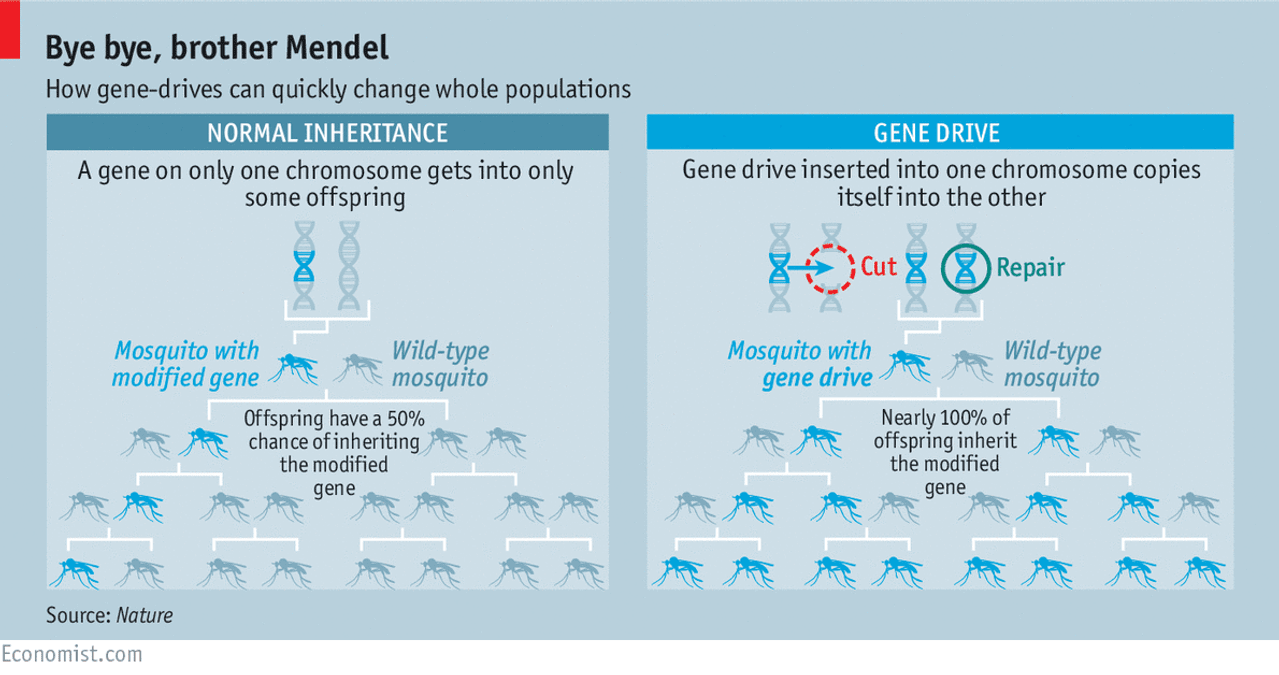
The implications for such a technology are obvious. Such a method could be used, for example, to cure a person of a genetic disease or more easily produce genetically enhanced crops for farmers. But there are even cleverer uses. Because the CRISPR-Cas9 system can be designed to be self-propagating, it can be used to force a gene into a population of animals, such as mosquitoes. If this system targets genes that are important for survival or reproduction, then once released, this "gene drive" would rapidly spread through the population, killing off mosquitoes. (See infographic from The Economist.)
Now, a team of researchers writing in the journal Nature Communications has shown that a gene drive can be used to suppress infection with cytomegalovirus, a type of herpesvirus. The underlying molecular mechanism of the gene drive is similar to others before it: A self-propagating chunk of DNA inserts itself into a gene that is important to the virus. In this case, the gene is UL23, which is needed for cytomegalovirus to avoid the human immune response.
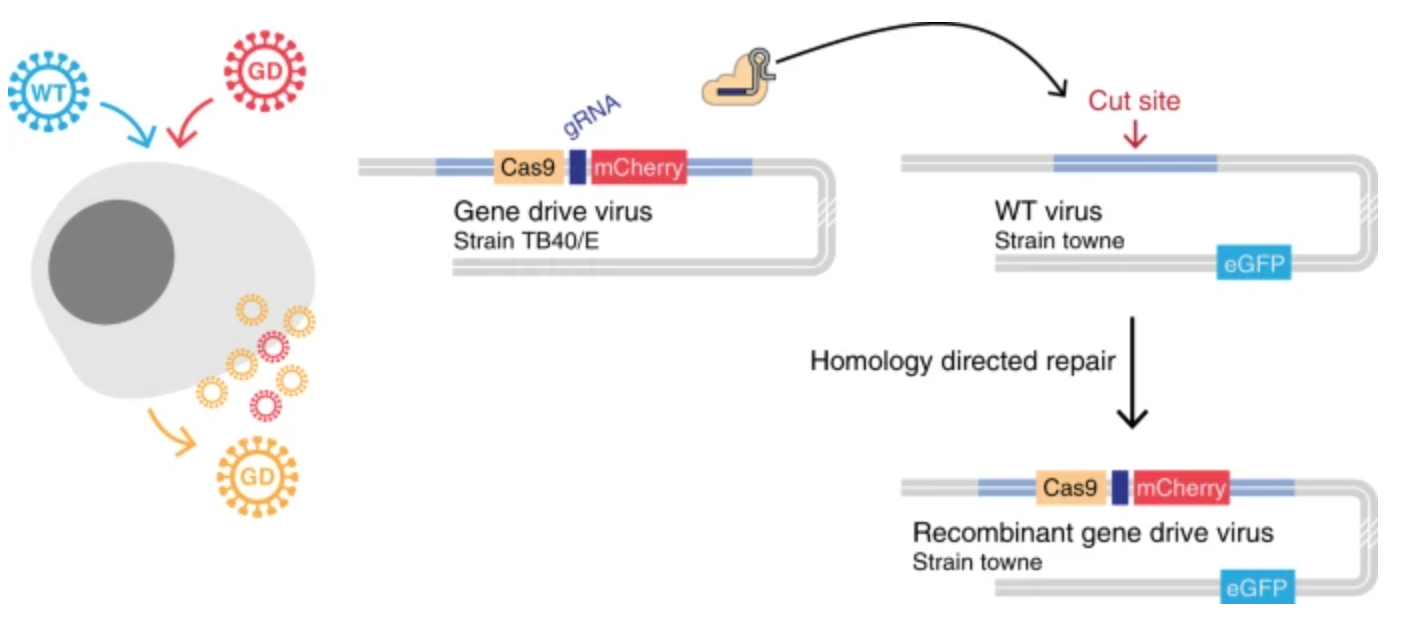
The researchers showed that when a cell is infected by both the normal virus (called "wildtype" or "WT") and the modified virus carrying a gene drive ("GD"), the gene drive was able to quickly and efficiently spread through the entire population, representing up to 95% of the final proportion of viruses. The end result is the suppression of viral infection (in cell culture, not in an animal model) because the gene drive virus lacks the important UL23 gene, which is needed for the virus to avoid a potent immune molecule known as interferon gamma (IFN-γ), which the authors added to the cell culture.
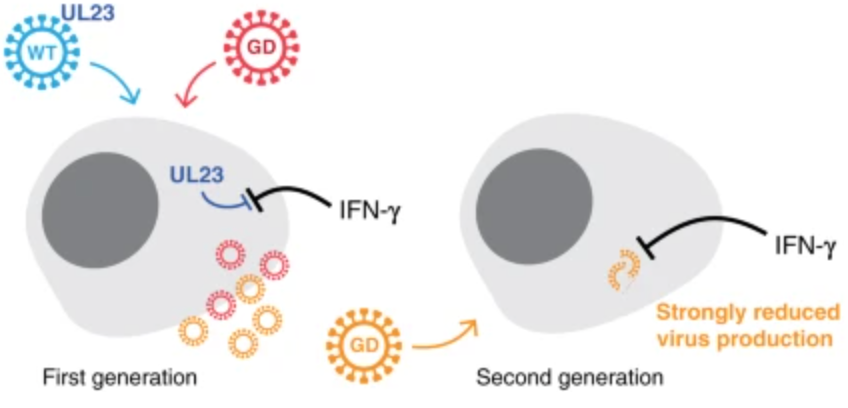
Could such a system work to treat viral infections in humans? Possibly. The authors note that a different gene (other than UL23) might need to be targeted, since lack of this gene is only fatal to the virus if IFN-γ is added to the cell culture. There are also concerns that a gene drive system could cause the viruses to mutate in various ways and may have unforeseen consequences.
Still, the technology is powerful and should be researched further. The coronavirus pandemic reminds us that we want to have multiple weapons in the public health arsenal should we be confronted with another life-threatening microbe.
Source: Walter, M., Verdin, E. Viral gene drive in herpesviruses. Nat Commun 11, 4884 (2020). Published: 28-Sept-2020. DOI: 10.1038/s41467-020-18678-0