Unlike stopping a pathogen with an antibiotic or antiviral drug, a process that is simple and well-understood, getting a handle on the seemingly impossible complexity of cancer cannot be more different. There are so many pathways and processes involved that even cancer researchers have a difficult time keeping it all straight. (1).
For example, it is well known that a crucial factor in the lethality of cancer kill is metastasis – the spread of cancerous cells (via blood vessels, the lymphatic system, etc.) from a primary tumor to other sites in the body, where they seed and grow new tumors. It is a process that is both hugely important and poorly understood; it has long been assumed that metastasis is restricted to cancer cells. Until now.
A new article in the journal Nature Genetics challenges everything we thought we knew about metastasis: It occurs in healthy cells too. In what could be a groundbreaking step in understanding and ultimately taming cancer, Drs. Richard Gilbertson, Eric Rahrmann, and colleagues at the Cancer Research UK (CRUK) Cambridge Institute studied the movement of both cancerous and healthy cells from one organ to another. They refer to the movement of healthy cells as a "collaborating machine," suggesting that this process plays a critical part in maintaining a system of organ health. Before we get any further you should bear in mind that the research was done in mice and there are a number of sayings about mouse models of cancer in oncology research (something I did during career #1) that sound pretty much like this:
“The history of cancer research has been a history of curing cancer in the mouse." (2)
Dr. Richard Klausner in "Ethics of involving animals in research" Trop Parasitol. 2013 Jan-Jun; 3(1): 4–6. doi: 10.4103/2229-5070.113884
The authors acknowledge this (3).
The science
The Cambridge group identified a protein with the not-so-user-friendly name Sodium Leak Channel Non-selective Protein, mercifully abbreviated as NALCN. Ion channels are ubiquitous throughout the body where they act as on-off switches, depending on whether the channel is open or closed.
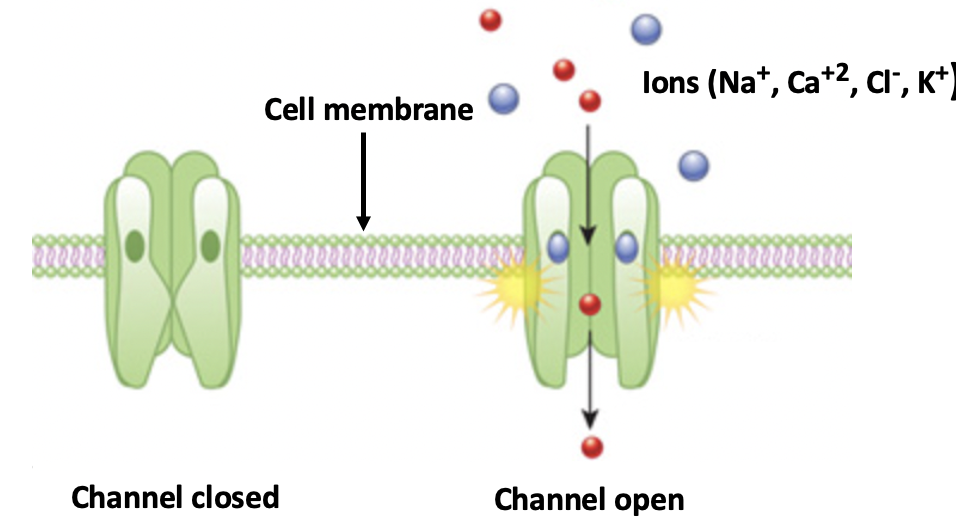
A simplified diagram of a typical ion channel. When closed (left) inorganic anions cannot penetrate the cell membrane. When open, ions freely flow through and elicit a biological response. Ion channels are physiological "on-off switches." Original image credit: Nature Education
The results are astounding
Chemically blocking the NALCN channel, which can be done by using gadolinium, increased the number of circulating tumor cells (CTCs) in the blood; more CTCs result in more metastatic "deposits." And these changes in the number of CTCs occurred even though the original tumors were unchanged. Clearly, the NALCN protein must play a significant part in transporting malignant cells from the tumor to other parts of the body. This discovery alone is ground-breaking. Then things got interesting.
Using genetic modification, the scientists were about to create healthy mice (no cancer) that lacked the NALCN gene (and therefore were unable to make the ion channels) – a process called gene knockout) – the group found that healthy cells started migrating from one organ to another. These findings suggest that NALCN plays a part in both metastasis and normal cell physiology. This was a jaw-dropping moment and another example of how cancer cells "hijack" normal cellular mechanisms in promoting their spread.
"These findings are among the most important to have come out of my lab for three decades...Not only have we identified one of the elusive drivers of metastasis, but we have also turned a commonly held understanding of this on its head, showing how cancer hijacks processes in healthy cells for its own gains. If validated through further research, this could have far-reaching implications for how we prevent cancer from spreading and allow us to manipulate this process to repair damaged organs."
Dr. R. Gilbertson in an interview with Interesting Engineering, 10/4/22
Summing it up
- The Cambridge group identified a ubiquitous ion channel called NALCN.
- NALCN is (at least partially) responsible for regulating cancer cell metastasis.
- When the NALCN channel was chemically blocked in mice with cancer metastasis (as measured by the flow of tumor cells) was turned up.
- In knockout mice, which were bred without the NALCN gene the same thing happened, but in healthy cells.
What this does and does not mean
- Assuming that this effect is reproducible (it really should be; this is a peer-reviewed, thorough, and convincing study in Nature) the universally accepted laws about metastasis will have to be rewritten. Or at least modified.
- Can it be shown that this discovery also applies to the corresponding human ion channel?
- If so, can NALCN be used as a screen for metastasis?
- Then NALCN will immediately become a target for drug discovery. Can drugs that keep the channel open be used to combat metastatic cancer be discovered?
- None of this will be happening anytime soon, even if everything works perfectly. Which never happens.
- Even further down the line is the possibility that gene therapy to introduce ion channels with open pores, which could (conceivably) be used to control or maybe even prevent metastasis.
- It's possible that we could be having an H. pylori moment in medical research. (4)
Finally, the importance of getting a handle on metastasis cannot be overstated:
“Once cancer has spread from the first tumour, it is harder to treat because we are looking at multiple sites in the body and working with new tumours that may be resistant to treatment. Discovering that a cancer has spread is always devastating news for patients and their families and so we are delighted to have supported this incredible research which may one day allow us to prevent metastasis and turn cancer into a much more survivable disease.”
Dr Catherine Elliott, Director, Cancer Research UK Cambridge Institute
NOTES:
(1) I'm not kidding. Here is a schematic showing the interactions of one kinase (an enzyme responsible for transferring phosphate groups from ATP to other biomolecules for the purposes of cell signaling) called MAP kinase. There are 538 kinases coded for in the human genome. Better get started.
The mitogen-activated protein kinase (MAPK) signaling pathway. Look away. Credit: Cusabio
(2) The entire quote is “The history of cancer research has been a history of curing cancer in the mouse. We have cured mice of cancer for decades and it simply didn't work in humans, we need to acknowledge the fact that use of animals will not make us better scientists, but bitter scientists”.
(3) "It is important to note that our observations are based on deleting Nalcn from mouse tissues, whereas NALCN in human cancers is affected predominantly by nonsynonymous mutations. Although our in silico modeling suggests strongly that these cancer-associated mutations close the NALCN channel, it will be important to demonstrate this functionally by modeling nonsynonymous Nalcn mutations in vivo. These studies should also include testing in patient-derived xenografts of gastric, colon and other cancers to confirm that NALCN regulates trafficking of human as well as mouse cells."
(4) This refers to the discovery that infections caused by H. pylori bacteria caused both gastric ulcers and stomach cancer, an idea that was originally scoffed at. The scoffing came to a screeching halt when Barry Marshall and Robin Warren won the 2005 Nobel Prize in Physiology or Medicine for their discovery.