My colleague Dr. Henry Miller is (rightfully) concerned about evolving Covid variants, especially how they may affect elderly people and others who are vulnerable to severe disease. Furthermore, increasing numbers of people are suffering from severe symptoms of Long Covid, which is manifesting itself in surprising and disturbing ways. Dr. Miller recently quoted Dr. Michael Osterholm, the Director of the Center for Infectious Disease Research and Policy at the University of Minnesota. (Osterholm was one of the infectious disease experts seen on TV at the onset of the pandemic.)
“By week[s] three and four [since the onset of his long Covid symptoms], the fatigue really set in worse than during the illness itself. And I started having memory loss. If you'd asked me, What's a Champagne and orange juice drink? I couldn't have thought of the word mimosa.”
Dr. Michael Osterholm
Given that Covid is not done with us I thought that it would be instructive to examine some of its inner workings, in particular, why is SARS-CoV-2, the virus that causes Covid, so different? It's largely because of its ease of mutation, something most of us know by now. What most of us don't know is that some of this can be explained by some rather simple chemistry (more on this later).
Rapid mutation of RNA viruses
The primary reason for rapid RNA viral mutation is an error – the incorporation of the wrong nucleobase into newly forming RNA and also the absence of enzymes to repair the error, such as those found in DNA. This results in a mutated protein, something I wrote about in 2021 when discussing how such errors can cause single amino acid changes in Covid spike proteins and how this can result in new variants or subvariants of the virus.
But there's another reason (1) that RNA viruses are less stable and more prone to mutation than DNA viruses and anyone who has made it it through a first-year organic chemistry course (as if that's so easy) should understand at least some of this. This is because, at the molecular level, one difference between stable DNA and unstable RNA comes down to a single oxygen atom. Prepare yourselves for a lesson in organic chemistry. Which means waking up Steve and Irving for another episode of...

Steve (left) and Irving, your eternal hosts of The Dreaded Chemistry Lesson From Hell®, seem to think that disturbing their nap for this particular episode is a waste of their time. Like they have any place better to go?
Sorry about the chemistry. But read it anyhow.
What is going on is a fundamental, well-known chemical reaction called ester hydrolysis – the breaking of an ester bond by water, producing a carboxylic acid and an alcohol (Figure 1)
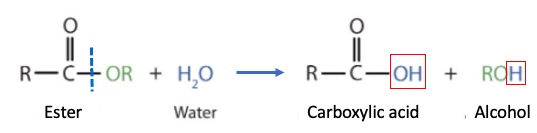
Figure 1. Hydrolysis of an ester consists of water adding to the ester bond and displacing an alcohol (R-OH), forming a carboxylic acid. The red boxes show the original water molecule. The blue hatch line shows the bond that is broken in the reaction.
Phosphate esters, the backbone of DNA and RNA, can also be hydrolyzed. The process (Figure 2) is conceptually identical to that of esters.
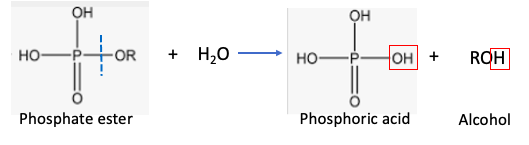
Figure 2. Hydrolysis of a phosphate ester. The red squares show the original water molecule. The blue hatch line shows the bond that is broken in the reaction.
What does any of this have to do with the instability of RNA and viral mutation? Time to roll out the chemistry. Sorry.
Anchimeric assistance (Neighboring group participation) Causes RNA Autocleavage
For reasons best left unstated, when a hydroxyl group is either five or six atoms away from an ester or phosphate these groups become much more reactive and break down more easily. This phenomenon is called anchimeric assistance (or neighboring group participation) and its effect is significant (Figure 3).
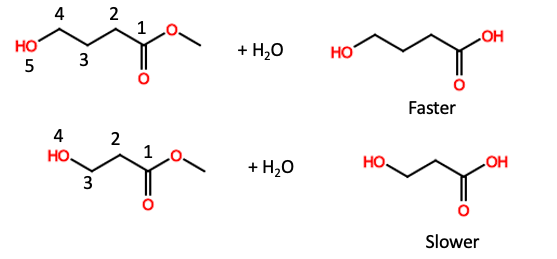
Figure 3. Comparative hydrolysis rates of two esters. (Top) The hydroxyl group at position 5 of the ester hydrolyzes (breaks down) quickly. (Bottom) When the hydroxy group is at position 4 of the ester the reaction is slower.
Figure 4 demonstrates the concept of neighboring group participation, but why does one carbon atom make a difference? It makes no sense. Except it does. This is one reason people hate organic chemistry. For just about every rule there is an exception. Here's one of them.
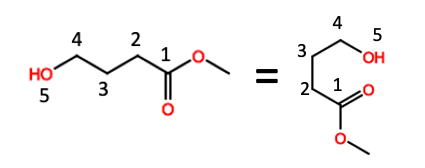
Figure 4. Methyl 4-hydroxybutyrate illustrated in two different conformations. Things might get ugly right about now. Good luck.
In Figure 4, the two figures are simply different representations of the same molecule. They are drawn in different configurations (for a reason), but it's still the same molecule.
Now you can see (Figure 5) why I've drawn the molecule this way. When the hydroxyl (OH) group is 5 atoms from the carbonyl group it is within perfect bonding distance to react with the carbonyl (C=O) group. By contrast, when the two groups are 4 atoms apart they are not at an optimal bonding distance and the hydroxyl group does not interact with the carbonyl group.
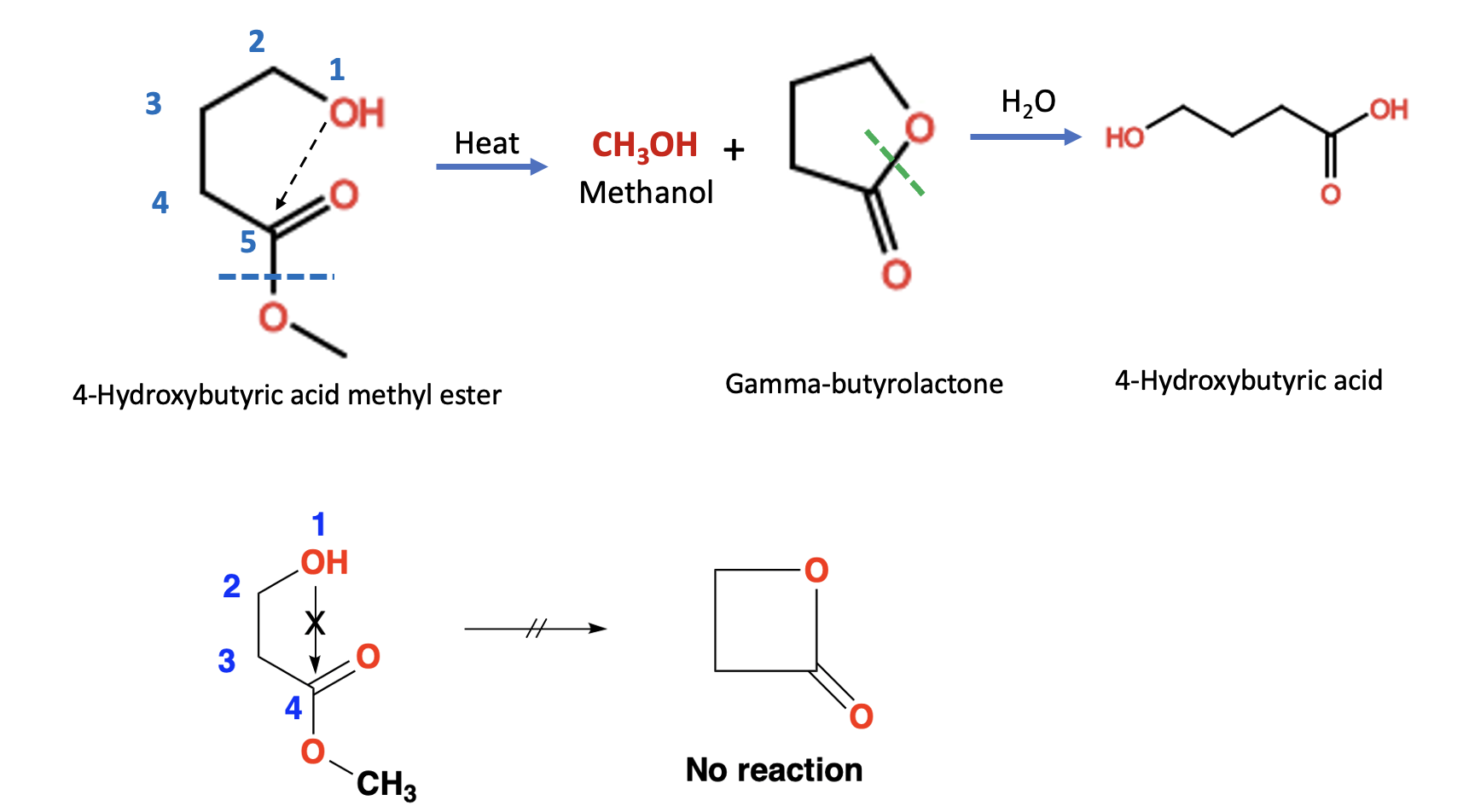
Figure 5. (Top) The reaction of 4-hydroxybutyric acid methyl ester to gamma-butyrolactone is promoted by the hydroxyl group 5 atoms away. First, the 4-hydroxybutyric acid methyl ester reacts with itself to form gamma-butyrolactone, which then reacts with water (green hatch line) to complete the hydrolysis reaction. This is an example of neighboring group participation. (Bottom) Removing one carbon from the top example makes a big difference. The hydroxyl and ester groups are four atoms apart - not an ideal binding distance. Consequently, heating the molecule does not result in the formation of the corresponding four-membered lactone.
What does this have to do with the instability of RNA?
A lot. Here's why. It's anachiometric assistance again, this time with phosphorous instead of carbon (Figure 6). This effect promotes the autocleavage of RNA, explaining its instability.
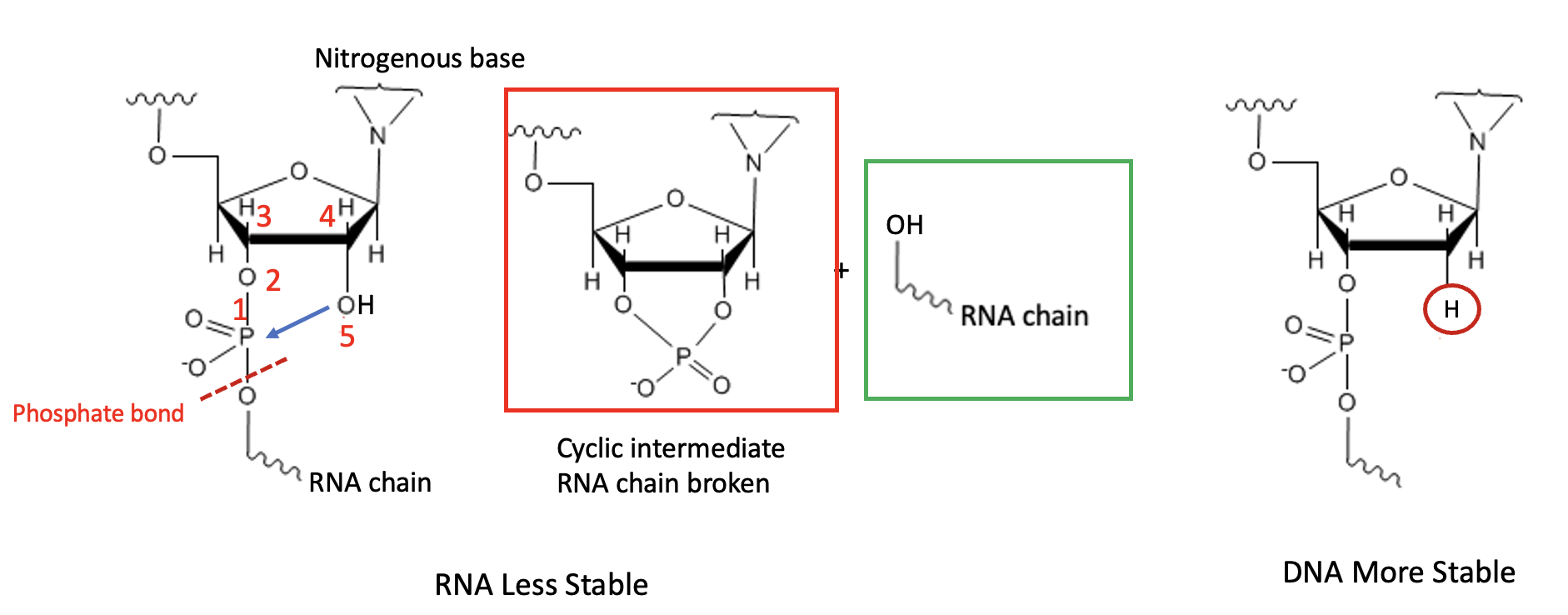
Figure 6. Autocleavage of RNA. (Left) As in Figure 5, RNA has a hydroxyl group that is 5 atoms (just the right bonding distance) from the phosphate group (blue arrow) that holds RNA (and DNA) together. This promotes a cyclization reaction, forming a transient cyclic phosphate (red box) in which the critical phosphate bond (red hatch line) has been broken. The resulting RNA fragment is in the green box. (Right) DNA has a hydrogen atom (red) circle in place of the hydroxyl group in RNA. Hydrogen does not participate in neighboring group participation and does not enhance this hydrolysis. This is why RNA is unstable relative to DNA.
So, it really just comes down to this:
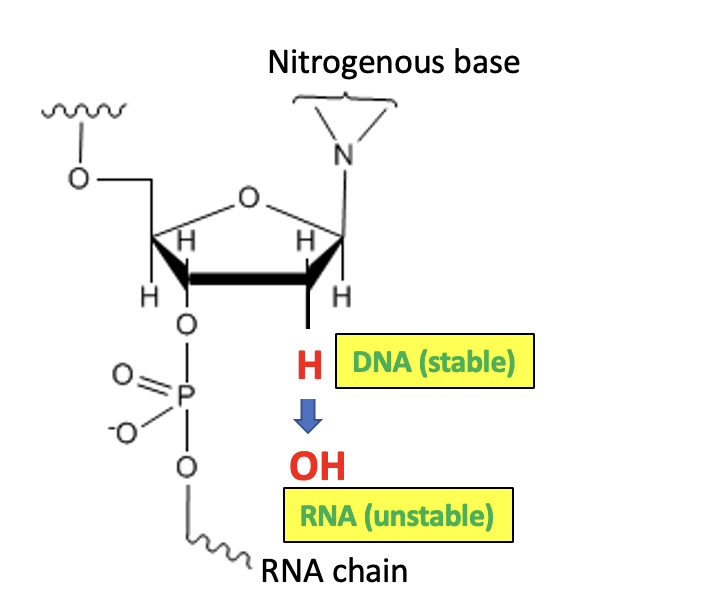
DNA differs from RNA by one oxygen atom, a seemingly trivial change in a huge molecule. But that's all it takes to make RNA less stable than DNA, which, in part, explains the seemingly countless variants and subvariants that spontaneously pop up all over the world. Like life itself, it's all based on "simple" chemistry.
NOTE:
(1) ChatGPT provides a nice explanation of how RNA strand damage contributes to mutation. I didn't dare put this in the article for fear of hate mail. Or worse.
"The 2' hydroxyl group is directly involved in the RNA replication process, and its presence contributes to the higher mutation rates observed in RNA viruses compared to DNA viruses. Here's how the 2' hydroxyl group can influence mutation...The combination of the error-prone replication and the lack of proofreading results in a higher mutation rate in RNA viruses. Mutations can accumulate rapidly in the viral genome, leading to the generation of diverse viral populations."