 The structure of HIV protease. Source: The Lancet
The structure of HIV protease. Source: The Lancet
Given the complexity of HIV/AIDS as well as the enormous toll it has taken on the world, the "culprit" that has enabled this catastrophe to take place is astonishingly simple — one molecule of water. Without it, there would be no such disease. Here's why:
Many viruses (including HIV) operate like this: 1. They enter the host cell. 2. Then they "hijack" the machinery that is normally used by the host cell to keep functioning. 3. Once the virus takes control of the host cell, the cell becomes a very efficient "virus factory," and starts making enormous quantities of viral genetic material and protein. 4. The viral proteins are not formed individually, but rather in one long strand, called a polyprotein. The polyprotein contains all the individual proteins that are required to build new viruses, but they are bound together.
Because of this, the polyprotein is not functional. It needs to be cut (processed) into a number — usually between 5 and 10 — of smaller proteins, which are functional, and are all necessary to build new virus particles. This cutting step (technically, breaking of a chemical bond) is called maturation (1), and is carried out by enzymes called proteases. If the action of the protease is blocked, the virus cannot replicate.
(A simple analogy for maturation is a watermelon. It cannot be eaten whole. To become edible, it must be cut up.)
It is a molecule of water does the cutting, but that water needs help from the protease enzyme, which holds the water molecule in the exact right position so it can do its job(2).
This can be illustrated by the two X-ray crystallography images below:
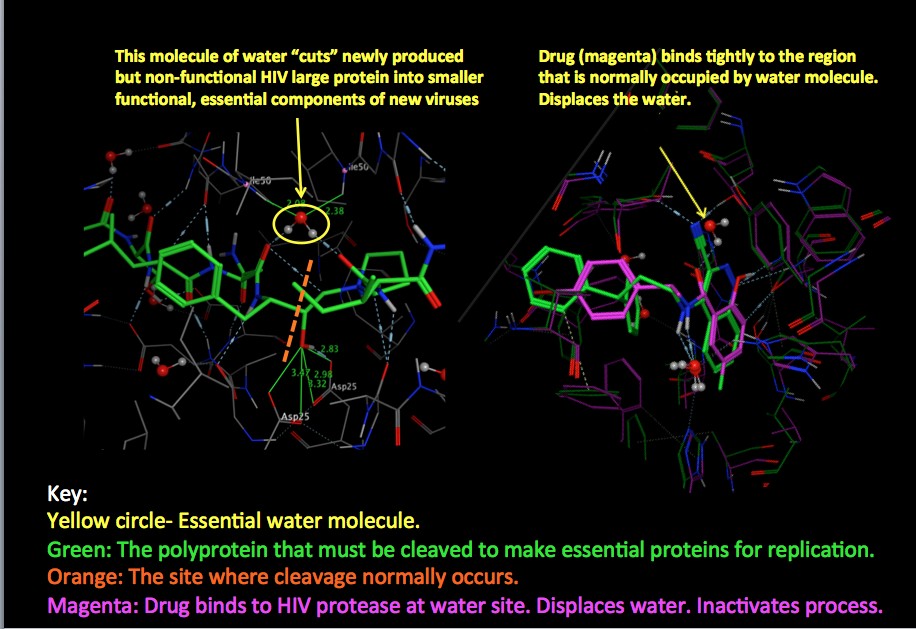
Source: Protein Data Bank
On the left is "business as usual." The polyprotein (3) is shown in green, and the orange line shows exactly where the cutting takes place. The protease holds the water molecule (yellow circle) in the perfect position to cut the polyprotein (4). (The faint colored lines in the background are part of the protease enzyme.)
On the right, things are quite different. The magenta is an HIV protease inhibitor (PI). PIs work by tightly binding to the active site (5) of the protease, and block the water molecule from occupying the required position. (Note that when superimposed, the overlap of magenta and green is good. This means that the inhibitor fits nicely into this space and will probably work well.) So, when the action of the protease is blocked, does it make a difference? It certainly does.
The first drug to effectively inhibit HIV protease, Saquinavir, was discovered by Roche. It was approved by the FDA in 1995. Several years ago when chemistry über blogger Derek Lowe asked his readers what they thought was most important drug in history, a good number of them said Saquinavir. They may have a point. Look what happened around that time:
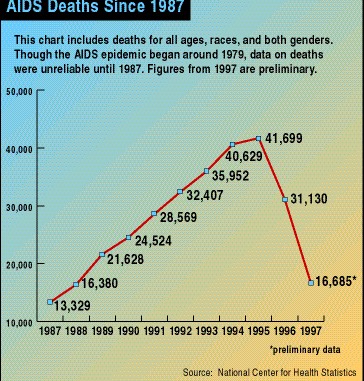
U.S. AIDS deaths by year. The number of deaths dropped for the first time ever in 1996
Source: National Center for Health Statistics
It is astonishing that 34 million people have died because of a chemical reaction that involves one molecule of water. Being able to understand this at the molecular level and use this knowledge to design a drug to combat this is pretty amazing as well.
Notes:
(1) There are multiple steps following maturation. I have intentionally omitted them.
(2) Proteases also use other mechanisms that activate the water molecule, but this is beyond the scope of this article.
(3) The green in this graphic represents only a tiny fraction of the polyprotein.
(4) Although it may look like the water molecule should be closer to the orange line, if it was, the cutting would not take place. The distance between the two is called "bond distance." The correct bond distance (the protease hold the water at just that) is essential for the cutting reaction.
(5) Active site refers to the part of an enzymes that performs a specific function.



