If there is a single lesson to be learned from three years of the pandemic, it's that if you're going to try to treat Covid with a drug, it had better be a direct-acting antiviral drug. (1)
To those of us who have been part of antiviral research campaigns (herpes simplex, HIV, and hepatitis C, in my case), this isn't news. Despite all the wishful thinking about repurposed drugs early on, none of them have panned out. And now, the antidepressant fluvoxamine, once promising, has joined hydroxychloroquine, ivermectin, and lopinavir (2), on the list of potential therapies that failed to show efficacy. (Dr. Katherine Seley-Radke, a professor of chemistry and biochemistry at the University of Maryland, Baltimore County, and an antiviral drug discovery expert, predicted this in a 2020 interview.)
I think it is a huge waste of time testing most of [the repurposed drugs]. Testing antiviral drugs? Yes, absolutely. Testing random drugs that aren’t direct-acting antivirals is just wasting valuable time that could be focused on finding a cure.
Dr. K. Seley-Radtke, 8/13/20
Based on early trials, fluvoxamine arguably showed the most potential as a Covid therapy. A 2020 trial that was published in JAMA showed a hint of efficacy for the drug, but the authors concluded that the trial size was not sufficient (152 patients) to evaluate its effectiveness properly.
But now, a randomized controlled clinical trial with almost 1300 patients answered the question:
Does 50 mg of fluvoxamine twice daily for 10 days, compared with placebo, shorten symptom duration among adult (aged ≥30 years) outpatients with symptomatic mild to moderate COVID-19?
McCarthy et. al., JAMA Network.
The answer is no, which is unfortunate because we are now down to two oral drugs, Paxlovid, and molnupiravir (aka Legavio), and one IV drug, remdesivir (aka Veklury). None of the monoclonal antibodies previously used to prevent severe disease in immune-compromised patients work on newer Omicron subvariants. In November, the FDA revoked emergency authorization for the lone survivor, bebtelovimab.
Fluvoxamine's lack of efficacy is not subtle:
=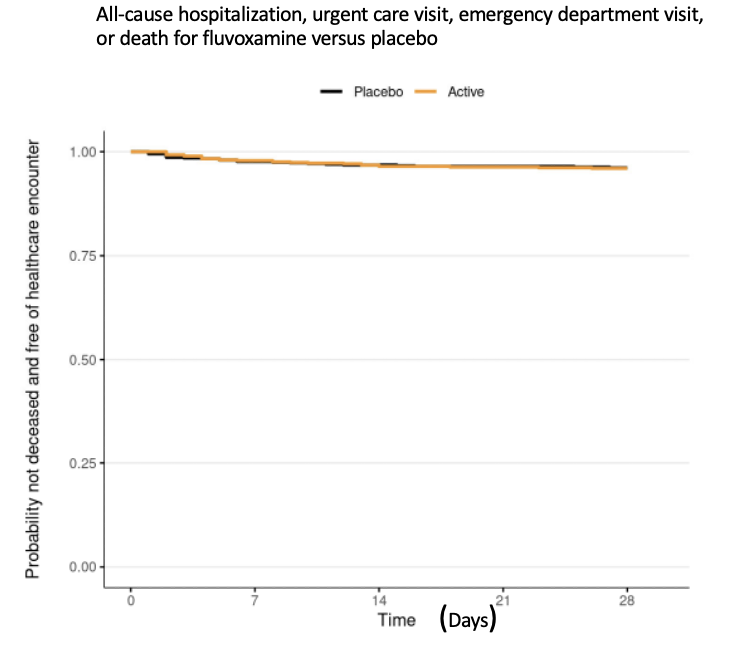
Source: McCarthy et. al., JAMA Network
The authors concluded:
Among outpatients with mild to moderate COVID-19, treatment with 50 mg of fluvoxamine twice daily for 10 days, compared with placebo, did not improve time to sustained recovery. These findings do not support the use of fluvoxamine at this dose and duration in patients with mild to moderate COVID-19.
Now what?
We need to keep looking for other, possibly better drugs, especially those that operate by a different mechanism of Paxlovid (nirmatrelvir/rotonavir) (3). Dr. Radtke adds:
"The strategy to tame Covid comes from the HIV/AIDS playbook: The discovery and development of multiple classes of direct-acting antiviral drugs that act by known, well-defined mechanisms. Perhaps the most pressing need is a nucleoside RNA polymerase inhibitor with a better safety and efficacy profile than molnupiravir, either as a replacement or in combination with Paxlovid as a way to reduce resistance to either drug. It is important to remember that HIV/AIDS became well-controlled only when "cocktails" of different drug classes became available. The same may prove true here."
NOTES:
(1) A direct-acting antiviral drug works by inhibiting a known, specific step in viral replication. Herpes drugs inhibit DNA synthesis. HIV drugs are grouped into classes that work the same way, for example, inhibiting maturation (protease inhibitors), RNA synthesis, entry into host cells, and others...
(2) Lopinavir made sense to try because it wasn't really a repurposed drug; it's an inhibitor of HIV protease and one of the approved AIDS drugs. Covid also has a protease enzyme, albeit one with a different size and mechanism of action; Nirmatrelvir, the protease inhibitor component in Paxlovid, was designed to inhibit the original SARS-CoV (the early 2000s) virus and also fit the protease binding site on SARS-CoV-2, which had a conserved binding site.
(3) The way to beat drug resistance is to treat a viral infection with at least two drugs that inhibit the virus by different mechanisms. It is more difficult for a virus to develop a fit strain that adapts to mutations in two independent targets.