
Insulin resistance is a hallmark of type 2 diabetes, and occurs in more than one type of body tissue. For example, insulin-resistant muscle will not respond normally when insulin directs it to take up glucose from the blood. And the liver of an insulin - resistant person won't stop its production and secretion of glucose into the blood as it should. As a result, blood glucose rises to abnormal, and possibly dangerous levels. In other words, a person becomes diabetic. Obesity is a major risk factor for diabetes and for insulin-resistant tissues, but how excess fat tissue causes insulin resistance isn't understood. A recent study in mice elucidates at least partially, a mechanism for how obesity instigates insulin resistance and type 2 diabetes.
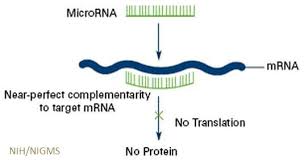
Writing in the journal Cell, Dr. Jerrold M. Olefsky from the University of California, San Diego and colleagues investigated the role of microRNAs (miRNAs) in modulating insulin resistance. miRNAs are short, around 22 base pairs, single-strand RNA molecules that do not code for proteins. Instead, they appear to modulate the expression of genes that do so, typically silencing them, as shown in the graphic below.
 The authors explain that obesity is a condition of chronic inflammation, which affects adipose tissue, liver, and perhaps muscle. As a result, so-called pro-inflammatory macrophages (a type of white blood cell) accumulate, especially in adipose tissue and liver. They suggested that factors produced and secreted from these cells (in particles called exomes) would be taken up by other tissues and could contribute to decreased insulin sensitivity. These factors were miRNAs, and the researchers tested their hypothesis in mice which were rendered obese by feeding them highly palatable foods.
The authors explain that obesity is a condition of chronic inflammation, which affects adipose tissue, liver, and perhaps muscle. As a result, so-called pro-inflammatory macrophages (a type of white blood cell) accumulate, especially in adipose tissue and liver. They suggested that factors produced and secreted from these cells (in particles called exomes) would be taken up by other tissues and could contribute to decreased insulin sensitivity. These factors were miRNAs, and the researchers tested their hypothesis in mice which were rendered obese by feeding them highly palatable foods.
To test this hypothesis, Dr. Olefsky and colleagues first determined that macrophage-secreted exosomes from adipose tissue of both lean and obese mice could be taken up by fat cells in vitro; this did occur. They then established that when macrophage-secreted exomes from fat mice were infused into lean mice, the lean mice then exhibited impaired insulin sensitivity compared to control animals. In addition, the infused mice also had higher blood glucose levels, as one would expect.
The group also used another mouse model, one that is unable to make the miRNAs, which are usually produced by adipose cells. When treated with exomes from these mice, fat cells from normal mice did not show the usual increase in insulin resistance, thus supporting the hypothesis that it was indeed the miRNAs that were inducing the insulin resistance.
In addition to the above, the investigators found that when obese, insulin-resistant mice were treated with the exomes from lean mice, their insulin resistance waned, making their glucose levels closer to those of normal animals.
These experiments support the hypothesis that the miRNAs derived from obese animals could impair insulin sensitivity and glucose homeostasis. The resulting hyperglycemia is diagnostic for diabetes, and of course, over the long term would result in serious complications.
We have come a long way from the time when adipose tissue was regarded as a static storage tissue for excess calories. It does store them, of course, but it is also an active player in the metabolic changes that are seen in the obese. Whether or not the results of the current experiments can be extrapolated directly to humans remains to be seen, but if so, it may be the case that understanding the role played by miRNAs from adipose tissue will lead to new means of treating obesity and perhaps avoiding obesity-linked diabetes.



