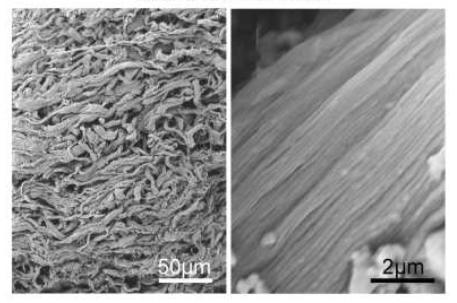
The discovery that wounds in the fetus can heal without scarring has prompted scientists to work on designing new biomaterials based on the properties of the fetal skin as promising regenerative strategies.
One key component of this property of fetal skin is thought to be the extracellular matrix (ECM) protein fibronectin. It is found in a particular form in the fetal skin called the fibrillar conformation.
A multi-institutional research group of engineers, chemists and biologists have now found a way to create a material similar to fibrillar fibronectin. And, when tested, it is highly effective in wound healing.
The researchers used rotary jet spinning (RJS) - a method that creates nanofibers. Using RJS, they could drive fibronectin fibrillogenesis (the development of the critical fine fibrils that make up the fibrillar form.) The fibronectin nanofiber scaffolds were then tested in a wound healing assay in mice.
When tested in a wound mouse model, the application of fibronectin nanofiber dressings to wounds did two things; 1) they accelerated wound closure and 2) significantly improved tissue restoration. They not only helped the recovery of dermal and epidermal tissues but also helped recover adipose (fat) tissue.
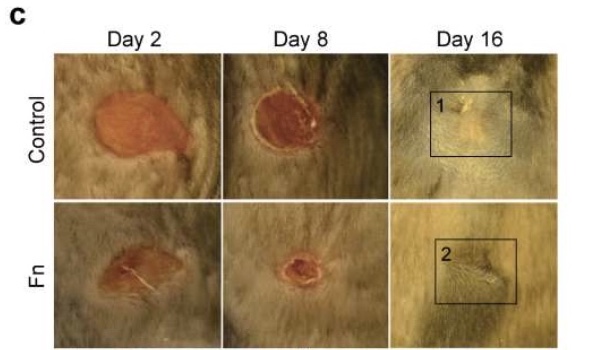
Shown in the figure below is a direct comparison of two wounds made in mice. The top row (control) shows the wound healing with no intervention. The bottom row (Fn) shows the wound healing with the added fibronectin treatment. It is easy to see that the wound treated with fibronectin healed faster and resulted in less scarring than the untreated wound.
This work shows the development of a new material that could be applied to a variety of regenerative medicine applications that would benefit from enhanced wound healing.
Source: Chantre CO, et al. Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model Volume 166, June 2018, Pages 96–108 Biomaterials. DOI: 10.1016/j.biomaterials.2018.03.006