
Carvone is terpene (See Essential Oils - Not What The Name Implies) which is found widely in plants, mostly in caraway seeds and spearmint leaves. These two sources are not accidental; they perfectly demonstrate how some molecules, although they are seemingly identical are subtly different when displayed in 3D. This seemingly strange phenomenon, which is called asymmetry or chirality, is actually quite logical. All you have to do is think about mirrors.
Here is the chemical structure of carvone in 2D:
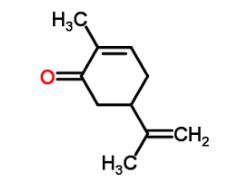
But there is a topic in organic chemistry that has been the demise of many pre-meds - stereochemistry, which exists because carbon, the element which makes organic chemicals organic, always has four bonds, either to itself or other atoms. Anytime that carbon atom is bound to four different substituents (a term that refers to atoms or groups of atoms) that carbon atom loses its symmetry (not surprisingly, these are called asymmetric carbon atoms). Once this happens that molecule can exist in two forms (Figure 1). This is because carbon is tetrahedral, not flat (1), at least most of the time (Figure 2).
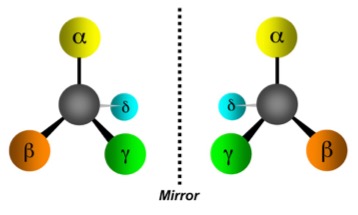
Figure 1. Two hypothetical molecules in which a carbon atom (gray) is bound to four different substituents (aka "things"). Although these have the same chemical formula they are not identical. The definition of identical molecules is those that can be superimposed on each other. These cannot. Rather, they are mirror images of each other. These are called enantiomers. Enantiomers almost always have different biological properties. Source: biopolyverse.
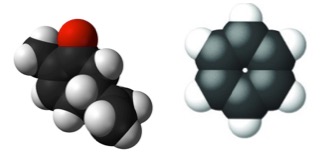
Figure 2. Space-filling models of carvone (Left) and benzene (Right). Flat molecules cannot be asymmetrical. Source: Lumen Learning
In the case of carvone, it is clear that there is one asymmetric carbon atom (Figure 2). So there are two enantiomers, both found in nature, that are mirror images of each other. One is labeled R, the other S. Don't ask.
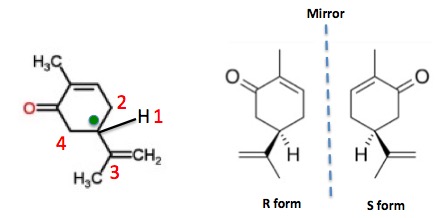
Figure 2. (Left) Carvone (drawn in 2D) contains one asymmetric carbon (green dot) because it is bound to 4 different "things." (Right) The two carvone enantiomers in a 3D representation (1).
This is where things get interesting (assuming that they haven't already been). And you'd damn well better say "Yes, Josh. This is already interesting" or my feelings are gonna be hurt.
If you open a bottle of the R-form you will smell spearmint. If you open a bottle of the S-form you will smell caraway seeds (think: rye bread). Same goes for taste. So it is no coincidence that S-carvone comes from spearmint plants and R-carvone comes from caraway seeds. Or you can buy them in a bottle. Sigma-Aldrich sells both (Figure 3).

Figure 3. Bottles of R- and S-Carvone. Both are commercially available. Source: chempics
Both forms of carvone are widely used as flavoring agents for different foods. They are both natural and artificial at the same time. It makes no difference. Confused? Feel free to read my mini-book about this. It's even on Amazon and hasn't gotten a single bad review! (2)
That's about it. Mirrors (without smoke) flavors, scents, stereochemistry, and molecular models. But no supermodels. We chemists can only do so much.
NOTES:
(1) Benzene-like molecules, which are also referred to as aromatic, are flat, which makes them symmetrical.
(2) Or a single good review



