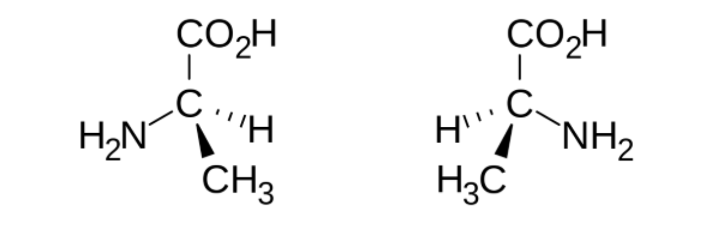Enantiomerism – the property of asymmetric molecules that look "the same" but are actually mirror images of each other – is one of the many topics, perhaps the most egregious, that drive organic chemistry students bats###t crazy. In case you're interested (or crazy), I've written about a simple molecule called carvone that, because of its asymmetry, exists in two enantiomeric (mirror image) forms. One smells and tastes like spearmint, and the other like caraway seeds.
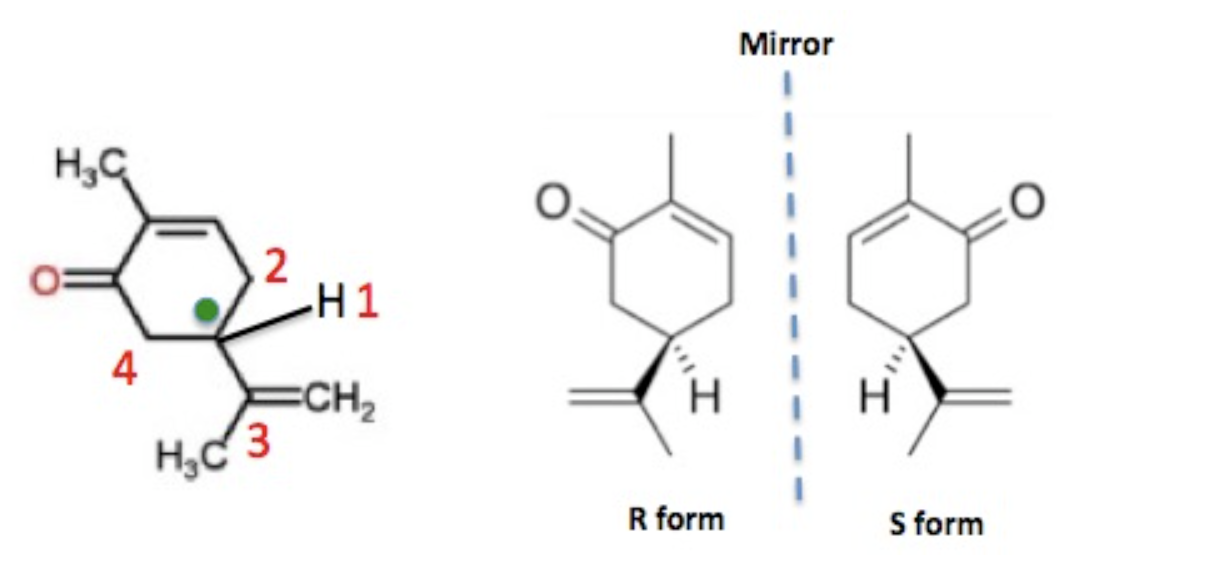
(Left) Carvone (drawn in 2D) contains one asymmetric carbon (green dot) because it is bound to 4 different "things." (Right) The two carvone enantiomers in a 3D representation, where it may or not be clear that they are mirror images of each other rather than identical.
Perhaps the most unfortunate example of the difference between the two enantiomers was thalidomide, the poster child of those who hate both drugs and the industry that discovers them. The drug was used widely in the 1950s (but not in the US) for its ability to help pregnant women cope with morning sickness. (It was also used as a sedative and sleep aid.) Unfortunately, thalidomide turned out to be the historical example used to explain that sometimes different enantiomers can have vastly different biological effects. There are countless articles about how one of the thalidomide enantiomers was safe, but the other caused severe birth defects. But they're all wrong. More on that later.
Most of the time, when a drug is asymmetric, one of its forms will be biologically active, and the other not. (The 50-50 mixture of enantiomers is called a racemate.) There are many examples of racemate drugs. One is the antidepressant Celexa (citalopram). The pill is a 50-50 mixture of the two enantiomers; one is called R and the other S. (There is not enough oil in Saudi Arabia to get me to explain this terminology.) There is also a different version of the drug escitalopram (Lexapro), which contains only the active S enantiomer. (The "es" at the beginning of the name stands for S.)
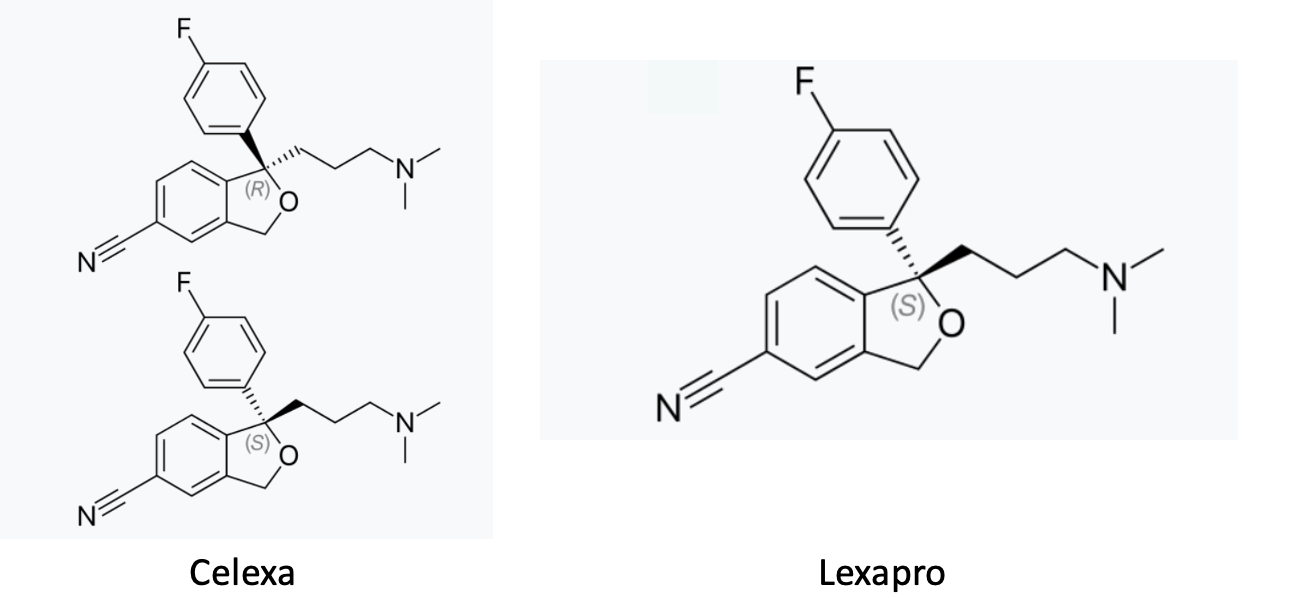
Celexa (citalopram) is a racemate – a 50-50 mixture of the inactive R form (1) and the active S form. Lexapro (escitalopram) contains only the S form.
Generally speaking, racemic drugs consist of one active form and the other carried along "for the ride" as if it's an inert substance. For example, the recommended doses for Lexapro (20 mg) and Celexa (40 mg) demonstrate that twice as much of Celexa is needed to get the same effect as the 20 mg dose of the active drug in Lexapro.
It is usually preferable (often required) that when a company is developing a new drug that is asymmetrical (1) that only the active component be used in the pill (an enantiopure drug). Why? Even though the R form of Celexa may not be an active antidepressant, this doesn't mean it can't act like a different drug; it can have other undesirable properties, such as toxicity or drug-drug interactions. So it is usually better to use only the active enantiomer in the pill rather than just double the dose and use the racemate. But not with thalidomide.
Thalidomide, a scientific urban legend
The concept that one of the thalidomide enantiomers was responsible for birth defects is still all over the Internet and not only from quacks or know-nothings; it comes from reputable organizations. There are more than 2,000 academic papers written about its mechanism of action.
Since thalidomide has a stereogenic carbon atom, it exists as two enantiomers. Tests with mice in 1961 suggested that only one enantiomer was teratogenic while the other possessed the therapeutic activity. Unfortunately, subsequent test with rabbits showed that both enantiomers had both activities.
Further research revealed that only the form on the right (the "R" form) was therapeutically active; the one on the left (the "S" form) was not only ineffective, it was the source of the birth defects!
Thalidomide is a chiral molecule and the drug that was marketed was a 50/50 mixture of left and right-handed molecules. While the left-handed molecule was effective, the right-handed one was highly toxic.
There are many such examples, all wrong.
Why the story is wrong
The problem is that thalidomide is unlike most racemates. Celexa is a racemate whose two forms are stable, whether inside a capsule or your body; one form has little or no bearing on the other. But thalidomide has a particular chemical property that makes this conventional wisdom incorrect. Just like other enantiomers, you can separate the racemate and put each form in its own bottle. But with thalidomide, things go to hell under certain conditions – like in your body – because of another chemical property with the ghastly name epimerization. My new best friend ChatGPT does a good job with this [emphasis mine]:
"Epimerization refers to a chemical process in which the stereochemistry of a molecule is changed at one or more chiral centers, resulting in the formation of an epimer."
This single sentence tells us why the explanations for the teratogenicity (the ability to cause birth defects) of one enantiomer but not the other of thalidomide are incorrect. The stereochemistry of the molecule is changed.
Uh oh. Here come chemical structures. You know what that means...

Your hosts, Steve (left) and Irving are raring to go with an especially hideous Dreaded Chemistry Lesson From Hell®
Hydrogen atoms (red dots in Figure 1) bonded to carbon atoms that are adjacent to a carbonyl group (carbon with a double bond to oxygen) are slightly acidic, so they can be removed by a base. One example, 2-methylcyclohexanone can exist as a racemic mixture of two enantiomers (R and S); because the molecule is asymmetrical (it has one carbon atom (green dot) bonded to four different groups). (For non-chemists this is rough going. You have to envision that the molecule is three-dimensional with the hydrogen atom pointed at you (the wedge bond) and the methyl group is pointed backward (the dotted bond); the molecule is not flat as it seems on paper. Nor can the R form be superimposed upon the S form, superimposition being the definition of identical.
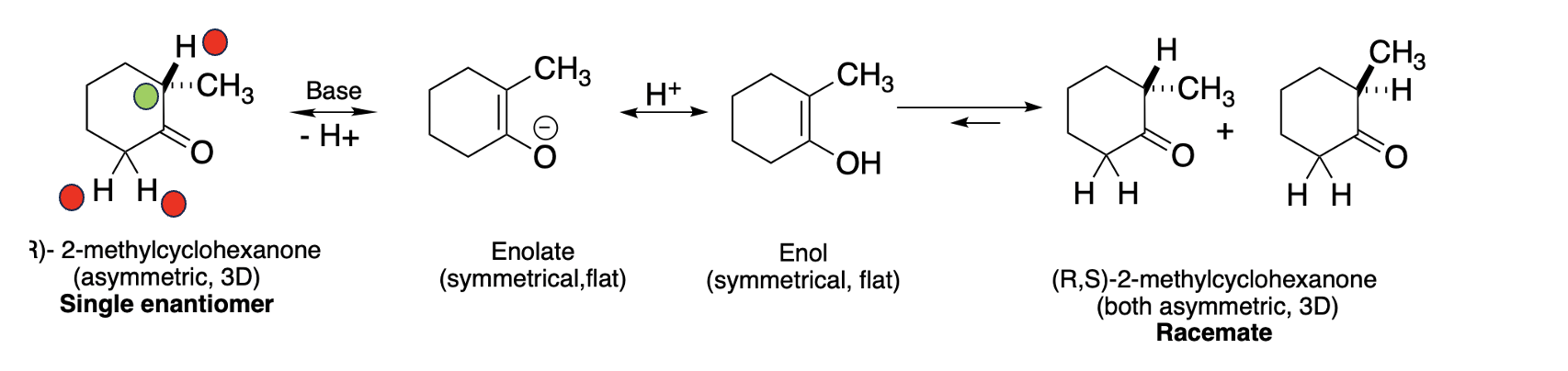
Figure 1. Racemization of R-2-methylcyclohexanone
(Please forgive me for the following.) If R-2-methylcyclohexane, a single enantiomer, is reacted with a base, the hydrogen atom (proton, H+) is removed, and the resulting anion (aka enolate) is flat and thus symmetrical. When the enolate is neutralized by another H+ ion, the result is an enol, which is also flat. (All of these steps are reversible.). Enols are in equilibrium with the favored keto form, so a rearrangement takes place where the hydrogen atom on oxygen is transferred back to the carbon atom next to the ketone from where it originally came. Now we're back at the starting molecule, but not quite. During the rearrangement the hydrogen atom returns to the carbon atom, it does so equally to the top and bottom of the flat enol, forming both the R and S forms in equal quantities. This is how epimerization/racemization takes place.
Thalidomide behaves in the same way. This is why it is now infamous.
It is the epimerization within the body is that makes thalidomide notable. As is the case with 2-methylcyclohexanone thalidomide has an acidic hydrogen atom (yellow arrow). Following the same pathway as in Figure 1, (R)-thalidomide – supposedly the "good" enantiomer – epimerizes to a mixture of "good" and "bad" drugs. But this is not strictly true. It would not matter if the pregnant women were given either enantiomer alone or a racemate of the two. The result would be the same: Two forms of one drug, one of which was responsible for the severe birth defects. If thalidomide simply contained a methyl group in place of the acidic hydrogen epimerization would have been impossible and thalidomide would just be a drug no one ever heard of.
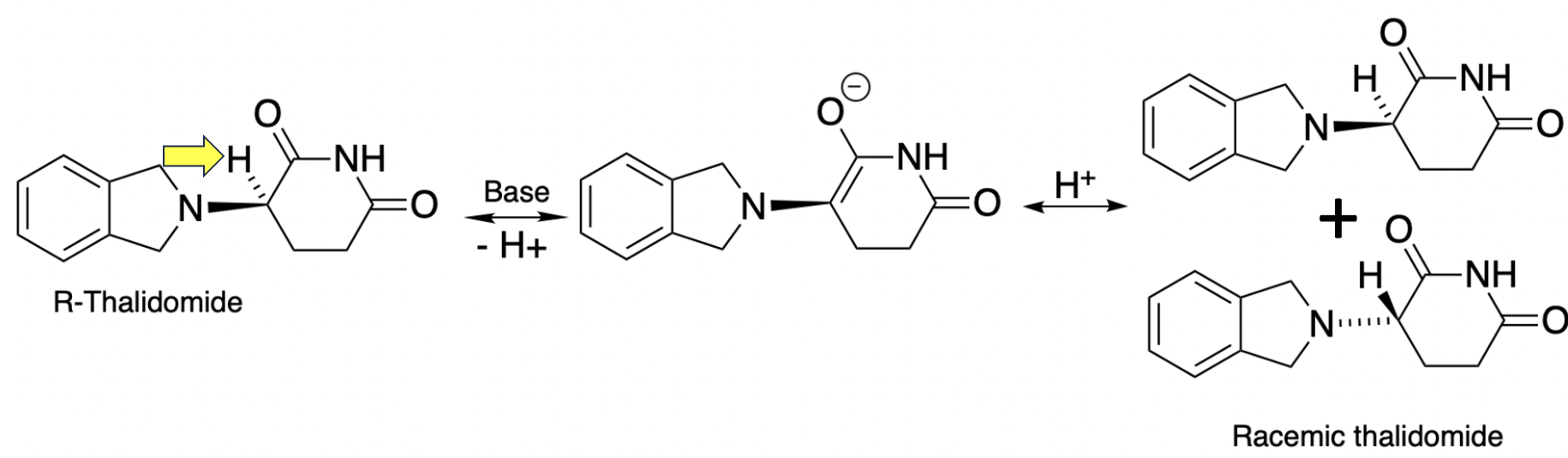
This is explained by the American Chemical Society:
Thalidomide exists in two mirror-image forms: it is a racemic mixture of (R)- and (S)-enantiomers. The (R)-enantiomer, shown in the figure, has sedative effects, whereas the (S)-isomer is teratogenic. Under biological conditions, the isomers interconvert, so separating the isomers before use is ineffective.
American Chemical Association Molecule of the Week, Sept, 2014
Thalidomide would never be approved today. Maybe.
Genotoxic screens, invented in the late 1960s, would have picked up the teratogenic activity of thalidomide immediately. It would have probably been dropped during the discovery process. Or, at the very least, its label (if approved) would contain strong warnings for women who might become pregnant, much like those for Accutane.
(The most famous genotoxic screen is the Ames Test (invented by long-time ACSH friend Dr. Bruce Ames)
But the thalidomide story doesn't end here. Despite its baggage, thalidomide has been repurposed for several conditions, including leprosy and cancer, for which it has received FDA approval.
NOTES:
(1) Perhaps this example will help some of you visualize the shape of an asymmetric chemical (tetrahedral). Shown are both forms of the amino acid alanine. They are enantiomers (mirror images).