The Frank R. Lautenberg Chemical Safety for the 21st Century Act amends the Toxic Substances Control Act (TSCA) and was signed into law June 22, 2016. It created a mandatory requirement for EPA to evaluate existing chemicals with clear and enforceable deadlines, to do so in a transparent fashion, and to do so using risk-based chemical assessments rather than rely on simple epidemiological correlations.
EPA selected the first 10 chemicals to undergo risk evaluation under the amended TSCA and to make those understandable for the public, the American Council on Science and Health is producing risk-based evaluations of each, which will then be compiled into a free downloadable book for consumers and policy makers.
What is N-Methylpyrrolidone?
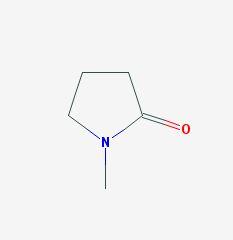
N-methylpyrrolidone (NMP), also known as N-methyl-2-pyrrolidone or 1-methyl-2-pyrrolidinone and colloquially as Methylpyrrolidone, is a colorless to slightly yellow liquid with a slight amine or “fishy” odor. It is heavier than water and has a flash point of 199 degrees Fahrenheit. NMP is an organic compound and its chemical structure is very simple, consisting of a 5-membered carbon ring where one part of the ring includes a nitrogen atom (molecular formula of C5H9NO, and in the image). For more see US National Library of Medicine’s Toxnet database, 2018a.
Exposure To N-Methylpyrrolidone
If NMP is released into the atmosphere, it will likely exist as a mixture of gas and liquid (or vapor). In air, NMP can be expected to dissolve into water droplets. However, since NMP has a low vapor pressure, this effectively limits its inhalation potential (EPA, 2018).
When released to water, NMP is not expected to bind to suspended solids or sediment in the water column and is not expected to readily evaporate from water (again because of its low vapor pressure). Because of this, NMP is expected to be highly mobile if released to soil, and it may evaporate (slowly) from soil surfaces or more likely migrate through soil and contaminate groundwater. In the environment, NMP is expected to be readily decomposed by bacteria or other living organisms and therefore is not expected to accumulate in the environment.
Humans may be exposed to NMP through inhalation and skin contact with liquid and vapor forms of NMP. Exposure may also occur through ingestion of mists that deposit in the nose, mouth and throat. Workers and occupational non-workers may be exposed to NMP in its manufacturing, production, formulation, handling, and application. Occupational non-users may also be exposed when they perform work in an area where NMP is present. Consumers may be exposed when using NMP-containing products, such as cleaning formulations, children’s toys, and textiles. Individuals may be exposed to NMP in the air from accidental releases from manufacturing, processing, distribution, and use, from its disposal in landfills where it may evaporate into the air or leach into groundwater, and from a small amount of volatilization of contaminated water during showering or bathing.
Populations living within or very near waste sites, or areas of heavy NMP use, might have an increased risk of exposure from contaminated media (air, water, or soil). However, no monitoring data have been identified for NMP in outdoor or indoor air, drinking water, surface water, sediment, or food. EPA estimated NMP concentration in ambient air to be approximately 0.41 mg/m3 using a model to estimate the exposure to populations located downwind of facilities reporting the highest NMP air releases based on 2015 Toxics Release Inventory (TRI) data (EPA, 2018).
Health Effects Of N-Methylpyrrolidone
NMP is well absorbed following inhalation, oral and dermal exposure in humans and animals. In laboratory animals, NMP is distributed throughout the body once absorbed, and is mostly metabolized to water-soluble compounds and excreted in the urine within 24 hours. In a limited number of humans, about two-thirds of the oral NMP dose administered and about one-quarter of the dose applied to the skin were excreted in the urine as metabolites and the rest as the parent compound.
Like exposure to any chemical, toxicity of NMP depends on the level to which one is exposed and the length of time of exposure. A well-documented case report that ruled out reasonable complicating factors provides some evidence that NMP may be toxic to the fetus. In this case report, a pregnant woman who was exposed to NMP by inhalation and dermal exposure lost her fetus at week 31 of pregnancy. Although the exposure levels were unknown, during week 16 of the pregnancy she reportedly cleaned up a spill of NMP that dissolved her latex gloves. She was ill for the next 4 days and experienced malaise, headache, nausea and vomiting. This case report appears to provide qualitative support for the NMP fetal toxicity that might come about from exposures to levels causing clinically evident toxicity in the mother.
In experimental animals, NMP has low toxicity following exposure to high levels over short time by inhalation, dermal exposure, and ingestion. When animals ingested NMP repeatedly at lower levels, adverse effects included changes in body weight, liver weight, neurotoxicity, and thymus atrophy. Long-term oral exposures in animals also caused increased incidence of large kidneys, kidneys diagnosed with chronic problems, fluid in the pleural cavity and small testes (Netherlands National Institute for Public Health and the Environment (RIVM), 2013).
More severe effects have also been noted when experimental animals were exposed by inhalation, which also includes dermal and oral uptake (from licking of the fur). The effects included irritation of the respiratory tract, reduced body weight gain, less bone marrow growth, testicular effects, necrosis in the thymus, spleen and lymph nodes, and mortality. Repeated application of high doses of NMP to the skin of animals resulted in mortality without other signs of systemic toxicity. (Systemic toxicity occurs as a result of absorption and distribution of a toxicant to a site distant from its entry point. In contrast, local toxicity occurs at the site of entry (e.g., lungs, stomach) of a toxicant into the body.)
No data exist in humans on the potential of NMP to cause tumors. In experimental animals, NMP was not carcinogenic, although results in a feeding study in mice showed liver tumors at the highest dose tested. The specific strain of mice used is regarded as very sensitive for inducing liver tumors that do not involve a substance binding to DNA. Such tumors are not normally considered relevant for humans, particularly when NMP is not mutagenic, i.e., it does not change the genetic material, usually DNA, of an organism. (RIVM, 2013).
Reproduction and developmental toxicity studies were conducted in experimental animals following inhalation, oral and dermal exposures. NMP did not have any effects on fertility in any of these studies. Nor were effects observed to the developing fetus at the highest exposure level tested following inhalation exposure. However, in an oral reproduction toxicity study, NMP caused developmental toxicity, as was evidenced by a decrease in fetal weight as well as malformations, and these effects were noted at doses lower than those that caused maternal toxicity, indicating that maternal toxicity did not cause the developmental toxicity. Dermal exposure also resulted in reduced body weight gain in the females and developmental effects, including fewer pups born, reduced body weights, delayed ossification of bones, and increased malformation. Unlike after oral exposure, however, these fetal effects occurred at the same dermal dose as in the maternal animals.
EPA (2015, 2017), RIVM (2013), and OECD (2007) have more information on the health effects of NMP.
Methylpyrrolidone Safe Levels
The federal and state governments develop regulations and recommendations to protect public health. Regulations and recommendations are often expressed as a safe or virtually safe level, that is, a level of a substance in air, water, soil, or food that is not expected to cause any adverse health effect, even in people who are sensitive to the chemical’s effects.
These safe levels are usually based on information from experiments with animals (usually rodents) at much higher levels of the chemicals than humans would typically encounter. The higher animal exposures are used to see which adverse health occur. Scientists then conjecture what the adverse effects could possibly be in humans at a lower level of exposure. Scientists can then estimate the level that will most likely protect humans. Sometimes these safe levels differ among federal and state organizations because they used different assumptions for human exposure, different animal studies, or employ methods that differ slightly. Other times these recommendations differ because new science develops that suggests different levels are toxic or safe. Recommendations and regulations are also updated periodically as more information becomes available.
The only organization that has derived a safe level for NMP is the International Programme on Chemical Safety (IPCS, 2001; NLM, 2018b) for the inhalation and oral routes. These safe levels are referred to as the tolerable daily intake (TDI) the European Commission Scientific Committee on Food regulatory equivalent to acceptable daily intake (ADI) for the oral route and tolerable concentration (TC) for the inhalation route. (1)
IPCS derived a TDI of 0.6 mg/kg body weight/day, based on for decrease in body weight and body weight gain and lower food consumption and food efficiency. The TC of 0.3 mg/m3 for NMP was based on nasal irritation, effects on hematopoietic and lymphatic organs, and mortality. In contrast, among the effects caused by NMP, EPA selected decreased fetal body weights as a key endpoint for use in the risk calculation for chronic exposure. While EPA did not derive a safe dose for NMP, it estimated an internal dose that corresponds to the administered dose that resulted in the decreased fetal body weights. EPA may compare this internal or external dose to human exposures to assess the risk posed by NMP or use a safe dose as described below. (2)
Why Is EPA Looking At This Under The Lautenberg Chemical Safety Act?
EPA scientists are currently looking at the likely routes of exposure to NMP in the environment and will be developing exposure scenarios, or pathways, of how the public comes into contact with NMP. These exposure pathways will then be studied by EPA by comparing the amount of NMP exposure in the pathway to its safe or virtually safe level, or to the internal dose associated with decreased fetal body weights (as noted above).
If human exposure in the pathway is at or below this safe or virtually safe level, then NMP exposure from the pathway is not considered to be a human health concern. Small excesses of the safe or virtually safe dose are seldom cause for concern since these safety levels are developed from conservative assumptions, including the use of safety factors that tend to exaggerate risk and exposure pathways that tend to exaggerate exposure.
If exposure is above this safe or virtually safe level, then the pathway might be considered as a possible health concern; several pathways may be added together to suggest a health concern.
In either event, regulations might be developed to lessen the exposure of NMP from this pathway(s).
Controversy Over N-Methylpyrrolidone
Health impacts of NMP include developmental and reproductive toxicity, neurotoxicity, immunotoxicity, and liver and kidney toxicity. Reproductive/developmental effects are sensitive endpoints for evaluating the human health risks associated with NMP exposure and EPA demonstrated in its 2014 NMP risk assessment that current levels of exposure to NMP-containing paint strippers pose an unacceptably high risk of adverse developmental toxicity (fetal effects) associated with acute and chronic exposure to female workers and consumers of childbearing age.
Developmental studies involve multiple exposures given on the order of 10-15 days and EPA (1991) considers these studies as relevant to single exposures because some developmental effects, such as fetal loss, may result from a single exposure at a critical period in fetal development. A critical effect is the first adverse effects or its known precursor, that occurs as a dose rate increases. It is assumed that if the critical effect is prevented, then all subsequent adverse effects are prevented.
In contrast, according to RIVM (2013), the critical effects following oral exposure in animals are reductions in body weight gain and food consumption.
EPA and other regulatory bodies have not classified NMP as to its carcinogenicity in humans. Because the most sensitive non-cancer effect of NMP is considered by some authorities to be decreased fetal body weights in laboratory animals, EPA used a physiologically based pharmacokinetic (PBPK) model to predict NMP doses across routes of exposure and from animals to humans. The model predicts an internal dose that is expected to be equivalent to the experimentally given dose. This internal dose is expected to cause the decreased fetal body weights in the laboratory animals and is used to compare to the human internal dose estimated from the occupational or consumer exposure pathway.
EPA is evaluating the pathways of exposure resulting from NMP consumer use. In the EPA (2015) risk assessment, dermal and inhalation exposures were assessed as the most likely exposure routes; however, there is a potential for oral exposure under some conditions of use. EPA also noted that consumers may purchase and use products primarily intended for commercial use. Therefore, waste streams associated with industrial and commercial use of NMP will also be a part of EPA’s evaluation.
EPA also expects to include, but not further analyze, exposures to humans, fish, aquatic invertebrates and algae from NMP releases to ambient surface water. EPA expects to include but not further analyze exposures to sediment-dwelling organisms during risk evaluation, as NMP is unlikely to accumulate in sediment. EPA does not plan to further analyze other land releases during risk evaluation, including those that may result from land application of biosolids, nor to further analyze NMP air releases or associated exposures to terrestrial wildlife, as inhalation exposure and bioaccumulation potential are expected to be low.
Likewise, EPA does not plan to further analyze human exposures that may result from inhalation of outdoor air containing NMP that was released from industrial and commercial facilities, since a first-tier screening analysis estimated a low potential exposure to populations located downwind from the facilities that reported the highest NMP air releases based on 2015 TRI data. Nor doe EPA plan to further analyze drinking water pathways, nor exposures associated with RCRA waste sites. This is because EPA has regular analytical processes to identify and evaluate drinking water contaminants of potential regulatory concern for public water systems under the Safe Drinking Water Act (SDWA) and has rules that govern the management and treatment, storage, and disposal of hazardous waste (i.e., Section 3004(a), Subtitle C).
Some of these exclusions may prove to be controversial.
NOTES:
(1) A TDI is an estimate of the amount of a substance in air, food or drinking water that can be taken in daily over a lifetime without appreciable health risk. ADI is a measure of the amount of a specific substance (originally applied for a food additive, later also for a residue of a veterinary drug or pesticide) in food or drinking water that can be ingested (orally) on a daily basis over a lifetime without an appreciable health risk. TDI is expressed, unlike the ADI, in mg/person, assuming a body weight of 60 kg.
(2) The ratio of an internal or external dose to the human exposure is referred to as a Margin of Exposure (MOE). This MOE can also be used as a threshold to determine the presence or absence of risk. To assess risks, the MOE is interpreted as a risk of concern if the MOE is less than the benchmark value (usually 100) (see EPA, 2018). On the other hand, the MOE indicates negligible concerns for adverse human health effects if the MOE exceeds this benchmark value. Typically, the larger the MOE, the more unlikely it is that an adverse effect would occur.
More analyses in our series on the Lautenberg Chemical Safety Act compounds:
ACSH Explains: What's The Story On Cyclic Aliphatic Bromides Cluster (HBCD)?
ACSH Explains: What's The Story On Carbon Tetrachloride?
ACSH Explains: What's The Story On Bromopropane?
ACSH Explains: What's The Story On Dioxane?
ACSH Explains: What's The Story On Trichloroethylene (TCE)?
ACSH Explains: What's The Story On Methylene Chloride (DCM)?
ACSH Explains: What's The Story On Asbestos?
REFERENCES:
EPA (US Environmental Protection Agency). 1991. Guidelines for Developmental Toxicity Risk Assessment. EPA/600/FR-91/001. Risk Assessment Forum, Washington, DC. http://www.epa.gov/raf/publications/pdfs/DEVTOX.pdf.
EPA (U.S. Environmental Protection Agency). 2015. TSCA Work Plan Chemical Risk Assessment. N-Methylpyrrolidone: Paint Stripper Use. Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100M55I.PDF?Dockey=P100M55I.PDF
EPA (U.S. Environmental Protection Agency). 2017. Scope of the Risk Evaluation for
N-Methylpyrrolidone (2-Pyrrolidinone, 1-Methyl-). Office of Chemical Safety and Pollution Prevention. Washington D.C. EPA Document# EPA-740-R1-7005. Available at: https://www.epa.gov/sites/production/files/2017-06/documents/nmp_scope_6...
EPA (U.S. Environmental Protection Agency). 2018. Problem Formulation of the Risk Evaluation for N-Methylpyrrolidone (2-Pyrrolidinone, 1-Methyl-). Office of Chemical Safety and Pollution Prevention. EPA Document# EPA-740-R1-7015. Available at https://www.epa.gov/sites/production/files/2018-06/documents/nmp_pf_05-3...
IPCS (International Programme on Chemical Safety). 2001. Concise International Chemical Assessment Document 35: n-Methyl-2-pyrrolidone. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization, Geneva, Switzerland. Available at http://www.who.int/ipcs/publications/cicad/en/cicad35.pdf
OECD (Organisation for Economic Co-operation and Development). (2007). SIDS Initial Assessment Report on 1-Methyl-2-pyrrolidone. Organization for Economic Cooperation and Development. http://webnet.oecd.org/hpv/ui/handler.axd?id=84daa4ac-feb7-4b5a-9839-206d17914e42
NLM (U.S. National Library of Medicine). 2018a. ChemIDplus: N-methylpyrrolidone. Toxnet database. Found at: https://chem.nlm.nih.gov/chemidplus/name/n-methylpyrrolidone
NLM (U.S. National Library of Medicine). 2018b. International Toxicity Estimates for Risk (ITER) database. N-methylpyrrolidone. Found at: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2
RIVM (National Institute for Public Health and the Environment (Netherlands)). 2013. Annex XV Restriction Report: Proposal for a Restriction. In RIVM, Bureau REACH. (Version 2). The Netherlands: National Institute for Public Health and the Environment (RIVM). Available at: https://echa.europa.eu/documents/10162/13641/nmp_annex_xv_report_en.pdf
Data written by
Michael Dourson, PhD, DABT, FATS, FSRA
Bernard Gadagbui, MS, PhD, DABT, ERT
Bethany Hansen, MA
all of Toxicology Excellence for Risk Assessment (TERA)