The Frank R. Lautenberg Chemical Safety for the 21st Century Act amends the Toxic Substances Control Act (TSCA) and was signed into law June 22, 2016. It created a mandatory requirement for EPA to evaluate existing chemicals with clear and enforceable deadlines, to do so in a transparent fashion, and to do so using risk-based chemical assessments rather than rely on simple epidemiological correlations.
EPA selected the first 10 chemicals to undergo risk evaluation under the amended TSCA and to make those understandable for the public, the American Council on Science and Health is producing risk-based evaluations of each, which will then be compiled into a free downloadable book for consumers.
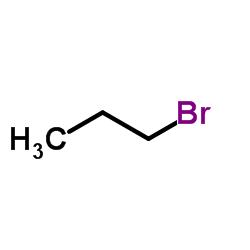
What is 1-Bromopropane?
As described by the US Agency for Toxic Substances and Disease Registry (ATSDR, 2017; 2018), the National Institute for Occupational Safety and Health (NIOSH, 2016), and US Environmental Protection Agency (EPA, 2016, 2018a), 1-bromopropane, also known as n-propyl bromide, is a colorless liquid. It has a sweet odor and quickly evaporates into the air when released to the environment. It is slightly soluble in water. 1-Bromopropane is used as a solvent in commercial and industrial applications, such as adhesive sprays, degreasing metals, plastics, and electronic components. It is also used in dry cleaning (including spot cleaning), asphalt production, aircraft maintenance, lubricants, and synthetic fiber manufacturing. It was originally used in the production of pesticides, flavors, fragrances, pharmaceuticals, and other chemicals. 1-Bromopropane is a synthetic chemical and is not known to occur naturally in the environment.
The chemical structure of 1-bromopropane is C3H7Br (NLM, 2018), as shown at the US National Library of Medicine’s Toxnet database (NLM, 2018). It is a type of organic chemical known as an alkyl halide in which a halogen atom, in this case bromine, is substituted for one of the hydrogen atoms on the terminal carbon atom of propane, a commonly used fuel to heat homes and cook food (North Carolina Department of Labor, 2013). This simple structure and the ability of our bodies to metabolize 1-bromopropane lead to its principle effect on the nervous system following inhalation exposure in both humans and animals.
Exposure To 1-Bromopropane
According to ATSDR (2017), the use of 1-bromopropane in manufacturing and commercial and industrial applications may result in its release into the air, water, sediment, and soil. Although 1-bromopropane is broken down quickly in air, it has been detected in the U.S. at very low levels of 0.14–0.16 parts per billion (ppb) (ATSDR, 2017). Unfortunately, little additional information was found on levels of 1-bromopropane that was released from manufacturing and processing facilities. There is also no reliable monitoring data for the levels of 1-bromopropane available in contaminated media at hazardous waste sites.
Some of the 1-bromopropane released into the soil or water is expected to also volatilize into air. However, it is not persistent in soil and in water is broken down by microbes. It is also not likely to bind to food and is not expected to concentrate within the food chain.
According to ATSDR (2017), EPA (2016) and NCDOL (2013), inhalation is the predominant route of consumer and occupational exposure to 1-bromopropane. Occupational exposure to 1-bromopropane occurs primarily from its use in spray adhesives, dry cleaning (including use as a spot cleaner), and degreasing (vapor, cold, and aerosol cleaning). Workers are expected to be the most highly exposed population. Occupational exposure to 1-bromopropane may also occur through skin contact, although exposure through ingestion and the eyes are other possible routes.
Individuals using consumer products containing substantial amounts of 1- bromopropane especially indoors may also have the potential for high exposure to this compound. ATSDR (2017) states that people may also be exposed to 1-bromopropane through the air when it is used during aerosol applications, specifically at locations in close proximity to facilities where it is used, processed, or manufactured. Another potential source of exposure of 1-bromopropane to individuals is vapor intrusion, although this has not been specifically confirmed. Vapor intrusion refers to the potential of volatile chemicals to migrate from groundwater contamination or contaminated soil into an overlying building. Vapor intrusion has been observed for several volatile organic chemicals that have similar properties and overlapping usage as 1-bromopropane (e.g., tetrachloroethylene in dry cleaning) (ATSDR, 2017).
People might also be exposed to 1-bromopropane in consumer products, including aerosol cleaning products, spot cleaners, and arts and craft spray glues. However, no consumer products were identified as containing 1-bromopropane in the U.S.
1-Bromopropane Health Effects
According to both ATSDR (2017) and EPA (2016), 1-bromopropane is well absorbed following inhalation and oral exposures, whereas lower absorption is expected following dermal exposures in both humans and animals. Absorbed 1-bromopropane is metabolized in two different ways. The balance between these two ways determines whether 1-bromopropane will form reactive or harmless breakdown products. Experimental animal studies indicate that 1-bromopropane is rapidly eliminated from the body by exhalation of 1-bromopropane, from metabolism to carbon dioxide that is then exhaled, and by urinary excretion of harmless 1-bromopropane byproducts.
Like exposure to any chemical, toxicity of 1-bromopropane depends on the level to which one is exposed and the length of time of exposure. ATSDR (2017), EPA (2016) and NIOSH (2016) have an abundance of health effect information on 1-bromopropane. According to these agencies, 1-bromopropane may cause health effects in workers following weeks, months, and years of inhalation and dermal exposure.
The major adverse effects of 1-bromopropane identified in humans occur in the nervous system. Case reports of workers occupationally exposed to 1-bromopropane for 2 weeks to 3 years have reported headache, dizziness, weakness, numbness in the lower extremities, ataxia (loss of
body movement), paresthesia (abnormal sensations in nerves---pins and needles), changes in mood, and motor and sensory impairments. Subtle effects have been reported at workplace air concentrations as low as about 1 ppm whereas irreversible loss of full control of bodily movements, inability to walk, and damage to nerves may occur at concentrations of 100 ppm and above. Studies in humans also suggest that 1-bromopropane may also be a respiratory tract irritant. The liver and kidneys may also be affected, however they are not as sensitive as the nervous system for 1-bromopropane exposure. Limited data exist to determine whether 1-bromopropane causes adverse reproductive and developmental effects in humans.
Results from experimental animal studies support the development of nervous system effects found from human exposures. For example, short-term exposure via inhalation to concentrations of 50 ppm and above resulted in changes in neurobehavior and the central and peripheral nervous systems. Ingestion of 1-bromopropane in animals for a shorter term also resulted in impaired learning and memory, sedation, and biochemical changes. In animals, 1-bromopropane exposure can also cause liver and kidney toxicity, adverse reproductive and developmental effects, as well as negative effects on the immune system. While there are no studies in humans that evaluated the potential of 1-bromopropane to cause cancer, longer-term inhalation exposures in animals have resulted in tumors in multiple organs or tissues including the skin, the large intestines, and the lungs.
1-Bromopropane Safe or Virtually Safe Levels
The federal and state governments develop regulations and recommendations to protect public health. Regulations and recommendations are often expressed as a safe or virtually safe level, that is, a level of a substance in air, water, soil, or food that is not expected to cause any adverse health effect, even in people who are sensitive to the chemical’s effects. These safe levels are usually based on information from experiments with animals (usually rodents) at much higher levels of the chemicals than humans would typically encounter. The higher animal exposures are used to see what the adverse health effects are. The scientists then conjecture what the adverse effects could possibly be in humans at a lower level of exposure. Scientists can then estimate the level that will likely protect humans, even those more sensitive to the 1-bromopropane.
Sometimes these safe levels differ among federal and state organizations because they used different assumptions for human exposure, different animal studies, or employ methods that differ slightly. At other times these recommendations differ because new science develops that suggests different levels are toxic or safe. Thus, it should be expected that recommendations and regulations are updated periodically as more information becomes available.
ATSDR (2017) has derived safe or virtually safe levels referred to as minimal risk levels (MRLs.) MRL is an estimate of daily human exposure to a substance that is likely to be without an appreciable risk of adverse effects (noncarcinogenic) over a specified duration of exposure. MRLs can be derived for acute, intermediate, and chronic duration exposures for inhalation and oral routes.
for inhalation and oral routes and for various exposure durations. For the inhalation route, the MRLs are 1 ppm for exposures of 14 days or less, 0.2 ppm for exposures for 15-364 days, and 0.02 ppm for exposures covering 365 days or more. For the oral route, ATSDR (2017) derived a value of 0.2 mg 1-bromopropane/kg-day for exposures for 14 days or less. No short- or long-term safe doses were derived because data were not sufficient to derive such safe levels.
EPA (2016) has not developed noncancer safe concentrations for any exposure route or duration. Instead, EPA created estimated hazard values, i.e., human equivalent concentrations (HEC.) Human Equivalent Concentration is the human concentration (for inhalation exposure) of an agent that is believed to induce the same magnitude of toxic effect as the experimental animal species concentration. For the oral route, this is referred to as the Human Equivalent Dose or HED. They compared HEC to the estimated inhalation exposures from occupational and consumer uses. HECs were derived for single and longer-term inhalation exposures only. These vales were 31 ppm or 10 ppm for single inhalation exposure of 1-bromopropane following occupational or consumer uses, respectively. EPA (2016) derived a range of longer-term inhalation HECs of 25 ppm to 150 ppm based on different effects following occupational uses only. The lowest inhalation HEC of 25 ppm was derived for nervous system effects.
Although ATSDR discusses the potential of a chemical to be carcinogenic, it does not currently assess cancer potency or perform cancer risk assessments. However, EPA (2016) derived a cancer inhalation unit risk (IUR) of 3 x 10-3 (or 3 persons for every 1000 people exposed) per ppm of exposure. Inhalation Unit Risk is an estimate of the increased cancer risk from inhalation exposure to a concentration of 1 µg/m3 for a lifetime. The IUR can be multiplied by an estimate of lifetime exposure (in µg/m3) to estimate the lifetime cancer risk.
Why Is EPA Looking At This Under The Lautenberg Chemical Safety Act?
EPA (2018b) is currently looking at the likely routes of exposure to 1-bromopropane in the environment and will be further developing exposure scenarios, or pathways, of how the public comes into contact with 1-bromopropane. These exposure pathways will then be studied by EPA scientists by comparing the amount of 1-bromopropane exposure in the pathway to its safe or virtually safe level. If human exposure in the pathway is at or below this safe or virtually safe level, then 1-bromopropane exposure from the pathway is not considered to be a human health concern. If exposure is above this safe or virtually safe level, then the pathway might be considered as a possible health concern; several pathways may be added together to suggest a health concern. In either event, regulations might be developed to lessen the exposure of 1-bromopropane from this pathway(s).
Small excesses of the safe or virtually safe dose are seldom cause for concern since these safety levels are developed from conservative assumptions, including the use of safety factors that tend to exaggerate risk and exposure pathways that tend to exaggerate exposure.
See EPA (2018b) for additional information related to the assessment of 1-bromopropane under the new Lautenberg Chemical Safety Act (LCSA).
Controversy Over 1-Bromopropane
EPA (2018b) interprets the mandates within the LCSA to conduct risk evaluations on current and prospective uses of 1-bromopropane for which manufacturing, processing, or distribution in commerce “is intended, known or reasonably foreseen.” 1-Bromopropane has a number of uses in these categories that are included in the current scope of EPA’s evaluation. However, EPA is excluding from its problem formulation the use of 1-bromopropane in agricultural products, adhesives and sealants associated with foam cushion manufacturing, and certain automotive care products such as engine degreasers and brake cleaners. The exclusion of some uses may be controversial.
As discussed above, studies in both humans and animals have identified the nervous system as a sensitive target of 1-bromopropane exposure following inhalation exposures. Other noncancer effects occur at higher concentration include effects on the liver, kidney, and the reproductive and developmental systems. For noncancer effects of 1-bromopropane exposure, EPA (2016) has used a range of these toxic effects for the safety assessment of longer-term exposures. For shorter-term exposures, EPA believes that developmental toxicity is appropriate for evaluating safety, based on the fact that such effects may occur from a single exposure during a critical period of development.
This is consistent with EPA’s Guidelines for Reproductive Toxicity Risk Assessment (EPA, 1996, 1991), which states that repeated exposure is not a necessary prerequisite for the manifestation of developmental toxicity. In both humans and animals, a relatively short critical window of vulnerability exists, and a single, high exposure could cause harm to a developing fetus. In addition, 1-bromopropane has a short half-life (half-life is the time required for the concentration of a substance in the body to decrease by half) and its metabolites are reactive toward cellular components (e.g., DNA and proteins) in several organs or tissues in the body.
Although no carcinogenicity studies were available in humans, EPA (2016) has noted that 1-bromopropane is a carcinogen that targets multiple sites in experimental animals following prolonged inhalation exposure. Based on this, 1-bromopropane is “reasonably anticipated to be a human carcinogen” (National Toxicology Program, 2013) and “possibly carcinogenic to humans (Group 2B) (International Agency for Research on Cancer, 2018). Although the exact mechanism of 1-bromopropane carcinogenesis is not known, EPA (2016) notes that the weight-of-evidence analysis for the cancer endpoint does not rule out mutation. In fact, several genotoxicity tests were positive using known or postulated metabolites of 1-bromopropane (EPA, 2016). Other authorities have indicated that 1-bromopropane causes cancer through adduct formation, oxidative stress, glutathione depletion, immunosuppression, and inflammation (European Chemicals Agency, 2016; ATSDR, 2017).
During the confirmation hearings of Dr. Michael Dourson, of the American Council on Science and Health Board of Scientific Advisors, to be Assistant Administrator of EPA’s Office of Chemical Safety and Pollution Prevention, it was claimed by political opponents that his nonprofit organization, Toxicology Excellence for Risk Assessment (TERA), proposed a weaker standard for 1-bromopropane. In reality, in 2004 occupation limits for 1-bromopropane differed by 16-fold between several organizations’ risk values. Dourson et al. had evaluated the underlying information and recommended an occupational exposure limit (OEL) of 20 ppm based on effects in newborns and their value was lower than EPA’s. An NTP study was conducted after the TERA assessment showing cancer findings. New evaluations based on the cancer study (new data) suggest lower limits could be considered.
More analyses in our series on the Lautenberg Chemical Safety Act compounds:
ACSH Explains: What's The Story On Dioxane?
ACSH Explains: What's The Story On Trichloroethylene (TCE)?
ACSH Explains: What's The Story On Methylene Chloride (DCM)?
ACSH Explains: What's The Story On Asbestos?
REFERENCES:
European Chemicals Agency (EChA). 2016. To support the DNEL setting for 1-bromopropane on its toxicity for reproduction, related to the use in Application for Authorisation. Helsinki. Available at: https://echa.europa.eu/documents/10162/21961120/1-bromopropane-consultant_en.pdf/c5c98a25-1a85-a13e-b796-e9935628c550.
International Agency for Research on Cancer (IARC). 2018. 1-Bromopropane. IARC Monographs 115. Available at: https://monographs.iarc.fr/wp-content/uploads/2018/06/mono115-01.pdf.
National Institute for Occupational Safety and Health (NIOSH). 2016. Criteria for a Recommended Standard: Occupational Exposure to 1-Bromopropane. Available at: https://www.cdc.gov/niosh/docket/review/docket057a/pdfs/ctd-1-bpcriteriadocument_final-012616.pdf.
National Toxicology Program (NTP). 2013. Report on carcinogens. Monograph for 1-bromopropane (Final) (pp. 1-168). (NIH Publication No. 13-5982). Research Triangle Park, NC. Available at: https://ntp.niehs.nih.gov/ntp/roc/thirteenth/monographs_final/1bromopropane_508.pdf
North Carolina Department of Labor (NCDOL). 2013. A Safety and Health Guide for 1-Bromopropane (n-Propyl Bromide). Available at: http://digital.ncdcr.gov/utils/getfile/collection/p16062coll9/id/148494/filename/157933.pdfpage/page/1
U.S. Agency for Toxic Substances and Disease Registry (ATSDR). 2017. Toxicological Profile for 1-Bromopropane. August. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp209.pdf.
U.S. Agency for Toxic Substances and Disease Registry (ATSDR). 2018. ToxGuideTM for 1-Bromopropane. Available at: https://www.atsdr.cdc.gov/toxguides/toxguide-209.pdf.
U.S. Environmental Protection Agency (EPA). 1996. Guidelines for Reproductive Toxicity Risk Assessment. (EPA/630/R-96/009). Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum. Available at: http://www.epa.gov/raf/publications/guidelinesreproductive-tox-risk-assessment.htm.
U.S. Environmental Protection Agency (EPA, 1991. Guidelines for Developmental Toxicity Risk Assessment. In US Environmental Protection Agency. (EPA/600/FR-91/001). Washington, DC: U.S. Environmental Protection Agency, Risk Assessment Forum. Available at: http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=23162.
U.S. Environmental Protection Agency (EPA). 2016. TSCA work plan chemical risk assessment: Peer review draft 1-bromopropane: (n-Propyl bromide) spray adhesives, dry cleaning, and degreasing uses. CASRN: 106-94-5 [EPA Report]. (EPA 740-R1-5001). Washington, DC. Available at: https://www.epa.gov/sites/production/files/2016-03/documents/1-bp_report_and_appendices_final.pdf.
U.S. Environmental Protection Agency. 2018a. Fact Sheet: 1-Bromopropane (1-BP). Available at: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-1-bromopropane-1-bp.
U.S. Environmental Protection Agency. 2018b. Problem Formulation of the Risk Evaluation for 1-Bromopropane. Office of Chemical Safety and Pollution Prevention. EPA Document # EPA- 740-R1-7019. Available at: https://www.epa.gov/sites/production/files/2018-06/documents/1bp_problem_formulation_05-31-18.pdf.
U.S. National Library of Medicine (NLM). 2018. ChemIDplus: 1-Bromopropane. Available at: https://chem.nlm.nih.gov/chemidplus/rn/106-94-5.
Written by
Bernard Gadagbui, MS, PhD, DABT, ERT
Bethany Hansen, MA
Michael Dourson, PhD, DABT, FATS, FSRA
all of Toxicology Excellence for Risk Assessment (TERA)