There is no doubt that stem cells have promising roles in medical care, and many clinics provide stem-cell therapies as their primary therapy or as a prominent offering in a buffet of services. They are not regulated by the FDA but use a halo of eminence and “evidence” to create the trust necessary to make the sale. Their methodology uses forms of medical misdirection we all experience in the news around COVID-19 therapies.
The study is a qualitative review of about one-third of website information for stem-cell clinics in the Southwest. [1] They sought to identify “tokens of scientific legitimacy” that provide a thin veneer of evidence.

The chart illustrates the credibility of scientific evidence presented, from registered clinical trials to medical qualifications, to testimonials from patients, celebrities and ratings. Most websites deliver their “veracity” indirectly, first, through the eminence of the practitioner, based upon board certification and association.
Eminence
Board certification is a reputational badge earned by practitioners. When I was in clinical practice, I was doubly boarded in general and vascular surgery. For various reasons, there has been a proliferation of boards certifying competence, so that among the 59 stem-cell clinics, they identified 34. The ten providers certified in “alternative medicine” involved nine different certifying boards – surely their criteria for training and competence must vary, otherwise why the proliferation. There was a similar proliferation of professional societies to which practitioners belonged. Membership in professional associations and board certifications were offered as evidence without evidence of the credibility of those societies or boards.
Second, through their use of scientific jargon that simultaneously elevates their speech while obscuring the meaning of their words. The description of stem cells and related procedures was written in medical textbooks' authoritative style and word-choice. But as the researchers point out, textbooks derive their authority from the credibility of their authors. On the websites, “it is not clear exactly who the authors are and what authority or expertise they bring to the table.”
Clinical Data
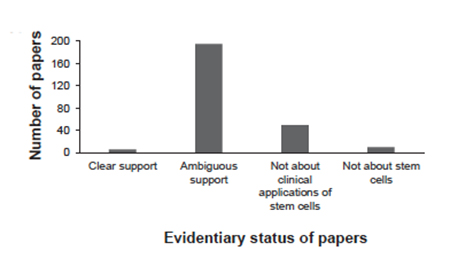 Like all medical treatments, the efficacy of the offered stem-cell therapies relies on the observed effects on people, in large groups or even individuals, as case reports involving one or three or four patients. Some offered citations and links to clinical trials or peer-reviewed papers – 261 papers in 170 different journals. [2] About a quarter offered a commentary or summary to those papers. The researchers spent the time necessary to read all those papers, to look at the cited evidence directly rather than by inference. Only 2% supported the statements being made. 75% were characterized as ambiguous – involving animals, techniques, and preparations not used by the clinics, different cell types, or medical conditions not being treated. Some even failed to show that stem-cell therapy was better than the control group, if a control group was even involved.
Like all medical treatments, the efficacy of the offered stem-cell therapies relies on the observed effects on people, in large groups or even individuals, as case reports involving one or three or four patients. Some offered citations and links to clinical trials or peer-reviewed papers – 261 papers in 170 different journals. [2] About a quarter offered a commentary or summary to those papers. The researchers spent the time necessary to read all those papers, to look at the cited evidence directly rather than by inference. Only 2% supported the statements being made. 75% were characterized as ambiguous – involving animals, techniques, and preparations not used by the clinics, different cell types, or medical conditions not being treated. Some even failed to show that stem-cell therapy was better than the control group, if a control group was even involved.
The researcher raised concerns about the publication of practitioners’ overall results. About 25% reported their patient outcomes. I have mixed feelings about this criticism. There is significant value in knowing how well your practitioner matches therapy to the patient and how well that treatment is performed – patient outcomes can capture that information. But what is unclear is the integrity of the data – who certifies that it is not “cherry-picked,” with poor results excluded? More commonly (71%), websites used a different form of outcome data, the testimonial.
There is a valid role for case reports or limited series to identify patterns that warrant attention and prompt further research. This useful role is lost when websites promote their version, testimonials, especially when heightened by the halo of celebrity. One cannot argue that these reports are false, but they are misleading in the presence of a greater truth that “past performance is no guarantee of future results.”
Why?
Within their discussion, researchers reveal an uncomfortable truth.
“…individuals are increasingly framed as consumers rather than patients, and are expected to take active roles in promoting and advocating for their own health .. [positioning] the prospective consumer as being in charge of their own health and challenges traditional sources of expert advice.”
It’s true. If I have been reduced to a provider, you have lost the protective title of patient and are now consumers. The care of your health is now a consumable good rather than a treasured relationship, with your lifestyle choices and physician-advisors. Sy Syms was correct, “An educated consumer is our best customer.” The difficulty is in becoming educated. Trusted resources are increasingly scarce on the Internet, crowded out by websites described by these researchers and many others.
We all are consumers of news. The day of the trusted news broadcaster, Walter Cronkite or Edward R. Murrow, has been replaced by the talking heads seeking to grab your attention. In any of the media forms, few have taken the time to read the primary science documents, let alone the citations those documents rely on and that they report. Fewer still have the background to translate the nuance and context. The same methods used to sell snake-oil stem cell therapies are used to feed us information on COVID-19. Just as with any marketing, “let the buyer beware.”
[1] Evidently, the Southwest, which includes Beverly Hills and Los Angeles, is a hot zone for stem cell clinics, with a third of the total found nationally.
[2] Only 10% were from “leading” journals, 1% were from “predatory” journals. So it is fair to say that the articles were more “me-too” than breaking science and not fluff meant to inflate a researcher’s CV.
Source: Weighing up the evidence used by direct-to-consumer stem cell businesses Stem Cell Reports DOI: 10.1016/j.stemcr.2021.10.007




