
Fat, in medicine, it is called adipose tissue, is the body’s primary energy reservoir, storing energy in the bountiful times, releasing it in the fallow. And while it appears to us to be unchanging, except perhaps to continue to grow, it is, in fact, a very plastic tissue responding to our energy environment, hormones, neural impulses, and even mechanical forces like compression.
There are several ways to categorize adipose tissue, beginning with its color.
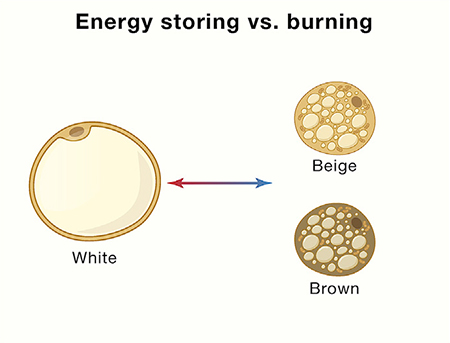 White fat – by far the most abundant, 80% is located just under our skin (subcutaneous); the rest deep within our abdomen (visceral) consists of single cells containing a drop of lipid. It is vital to our energy economy enabling a quick change from releasing energy to storing energy, ensuring “that other organs always have an adequate but not excessive level of energy.”
White fat – by far the most abundant, 80% is located just under our skin (subcutaneous); the rest deep within our abdomen (visceral) consists of single cells containing a drop of lipid. It is vital to our energy economy enabling a quick change from releasing energy to storing energy, ensuring “that other organs always have an adequate but not excessive level of energy.”
- Brown and Beige Fat – Brown and beige fat are a minority in adipose tissue containing many drops of lipid and many mitochondria, our true energy source. The unique quality of brown fat is that it can uncouple its metabolism to generate heat, termed thermogenesis. It is what keeps us warm when it is cold [1]. Unlike the brown fat deposits present at our birth, beige fat develops in white fat in response to cold exposure. It is adipose tissues switch-hitter able to go back and forth from white to brown – hence the term beige. Brown fat can suppress weight gain by increasing thermogenesis.
Lipogenesis and lipolysis
Lipogenesis refers to creating fats from excess nutrients, specifically carbohydrates, and protein. By far, the biggest driver of lipogenesis is insulin, which
- stimulates the uptake of glucose, the precursor for the formation of lipids
- while also suppressing the reduction of fats to energy molecules (lipolysis).
Additionally, lipogenesis creates “several lipid species with anti-inflammatory and insulin-sensitizing effects.” Lipolysis breaks down those fat stores into energy molecules, glycerol, and free fatty acids. It is stimulated by increased sympathetic nerve activity and leptin, a hormone that inhibits hunger. The balance between insulin and leptin might be thought of as your body’s energy thermostat, increasing the storage or burning of energy. In contrast to white fat, brown and beige fat are more regulated by our sympathetic nervous system. Brown fat is more densely innervated than white fat, and those neural connections increase with cold exposure. As we age, that innervation is lost to some degree, more so in women than men, which might help explain why older individuals, especially women, find a room “colder” than their male partners.
Fat expands and contracts
Adipose tissue “has an unparalleled ability to expand and contract compared with other organs.” It can range from 5% of body weight in the super lean to 70% in the morbidly obese. Women have slightly more adipose tissue than men. When we take in “excess” nutrients, our metabolism can either burn them off through exercise or thermogenesis or store them. Interestingly, white fat can safely store large amounts of fat, but it is limited by the size and number of adipose cells. The excess accumulates in other tissues.
“Notably, the site of adipose tissue expansion (into visceral or subcutaneous depots) and the mechanism of expansion, via increases in adipocyte number (hyperplasia) or size (hypertrophy), have profound impacts on metabolic health.”
This is the underlying reason why scientists may describe obese individuals as metabolically healthy and some normal-weight individuals as metabolically unhealthy. It is all about deep or visceral adipose tissue. As we gain weight, both our superficial and deep adipose stores increase, but men tend to create more deep, visceral adipose tissue – the adipose tissue associated with “adverse metabolic outcomes” – including inflammatory signaling and insulin resistance. The good news is that weight loss is associated with a greater loss of visceral as compared to subcutaneous fat.
Hypertrophy and Hyperplasia
The expansion of adipose tissue is fueled by two means. The fat cells may expand in size, hypertrophy, or number, hyperplasia. Adipose can grow in size within some limits, to the point of what might be described as moderate obesity. That, unfortunately, is a bit of a circular definition because greater degrees of obesity are termed “morbid” obesity because of the unfavorable metabolic changes. Larger adipocytes can and do produce inflammatory mediators; it is a matter of degree, let’s call moderate obesity 10-20 pounds overweight. For those losing weight, cell size reduces.
“Once adipose tissue becomes ‘‘full’’ and can no longer take up excess nutrients, ectopic lipid begins to accumulate in peripheral organs leading to metabolic decline.”
With hyperplasia, the total number of adipocytes increases, and this form of fat expansion “is linked with higher levels of adipose tissue inflammation, fibrosis, and hypoxia, along with poor metabolic health.” Weight loss does not reduce adipocyte numbers. As the authors write, “adipocyte number might function as a one-way ratchet, expanding in obesity, but not declining after weight loss.”
There is substantial variation in the places and types of fat that individuals accumulate. It is not fixed and can be modified by several factors.
- The total number of fat cells increases as we age, leveling off in adulthood. Unfortunately, obese children create more fat cells.
- The balance between nutrient intake and fat use
- The hormonal effects of androgens and estrogens. Androgens produce more fat around our abdomen and increased visceral fat – characteristic of men. Estrogens stimulate more fat around the pelvis and buttocks – characteristic of women. With the onset of menopause, women develop more “android” fat deposits.
“Genetic and pharmacological studies suggest that it is not hypertrophic adipocytes per se that drive systemic metabolic dysfunction, but rather a failure of adipose-tissue ‘‘expandability.’’ …hypertrophic adipocytes are a symptom more than a cause of dysfunctional adipose tissue.”
Healthy adipose cells are contained by a “loose mesh” called the extra-cellular matrix, which gives fat structural support and provides a milieu for molecular signaling. As adipose tissue expands, it can outstrip its blood supply, lowering the local oxygen levels and killing off adipocytes which release their lipid contents, causing more inflammation and subsequent fibrosis – a hardening of that loose mesh to a much tighter net. Adipocytes are “mechanosensitive,” fibrosis makes them dysfunctional, releasing more inflammatory mediators. And the tightened mesh makes it more difficult for adipocytes hypertrophy, tilting the balance towards more harmful hyperplasia. As the authors note, all of these changes “are continuous and mutually reinforcing, making it difficult to disentangle cause and effect.”
When we speak of inflammation, we must consider an essential member of our immune system, macrophages. Macrophages are specialized white blood cells that swallow up foreign materials, like bacteria and lipids released into the tissue by dying adipocytes. They reside, among other places in that extra-cellular matrix, the mesh holding fat cells. Obesity alters those macrophages, moving them from an “anti-inflammatory” to a “pro-inflammatory” state. These are the macrophages that come for the dying fat cells. It is interesting to speculate whether obesity’s creation of more pro-inflammatory immune cells also plays a role in obesity as a risk factor for poorer outcomes from infections, like COVID-19.
Key points
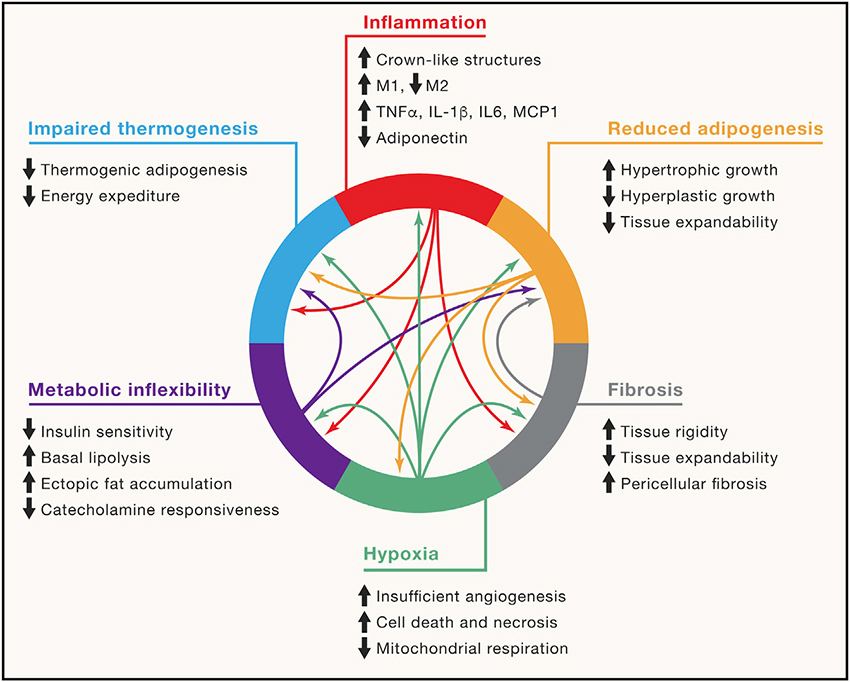
- Healthy white fat “exhibits extensive metabolic flexibility,” ensuring we have the energy we need.
- When we take it more than we need, white fat becomes less flexible. We develop insulin resistance and make more and larger fat cells. Those cells, in turn, can cause local loss of oxygen, resulting in inflammation, fibrosis and further reducing the ability of white fat to expand in a “healthy” manner.
- When we take in less than we need, through fasting or other calorie restrictions, obesity impairs signaling, and the normal mobilization of fat to provide energy is diminished.
- “Ultimately, these pathological changes impair the critical nutrient-buffering function of adipose tissue, leading to insulin resistance and metabolic disease.”
[1] It is the reason that you can find New Yorkers, among others, walking around in shorts in February.
Source: Adipose-tissue plasticity in health and disease Cell DOI: 10.1016/j.cell.2021.12.016



