As marijuana use proliferates, so does the science behind it. But unlike single psychotropic drugs such as oxycodone, Prozac, or LSD, the pharmacology of cannabis is far more complex. Why? Because more than 400 chemicals, many of which are psychotropic, have been identified in the plant. Many of these belong to the widely known cannabinoid class, but the plant also contains hundreds of naturally occurring volatile chemicals called terpenes (see below), which are partly responsible for the scent, flavor, and pharmacological properties of cannabis.
One of the more interesting members of this class is a structurally simple hydrocarbon called myrcene and it's thought to be partially responsible for the sedating effects of cannabis. But myrcene has multiple functions; it is found in minute quantities in a variety of herbs and spices (beer too) and has alleged biological properties that include:
- Analgesic
- Antioxidant
- Anti-inflammatory
- Antidiabetic
- Antibacterial
Then there's this
- Anticancer
- Along with this...

Huh? How can myrcene have both anticancer properties and also be a known carcinogen (1)? It's not an isolated instance once you enter Crazyfornia California's Prop 65 list of random carcinogenic chemicals. After all, only in CA can coffee both cause and cure cancer. (Also see Should California Put A Warning Label On Your Penis?) I don't want to dwell on the insanity of Prop 65, so the following photo should do the trick.

Death in Disney. Some Mickey Mouse science. Photo: Wikimedia Commons
What are terpenes?
I am reluctant to recruit Steve and Irving for another Dreaded Chemistry Lesson From Hell,® because they'd need to be paid overtime (Hell has a powerful teamster's union) – something that the cheapskates at ACSH simply refuse to do. This is an example of unethical behavior if you ask me because according to reliable sources like Andy Kolodny and Adriane Fugh-Berman, members of PROP (a different kind of Hell), claim ACSH is bursting at the seams with all that corporate money that pours into our filthy coffers. So, why not pay the poor guys?

Steve (left) and Irving bemoan the fact that ACSH, despite dripping in filthy corporate cash, refuses to pay them overtime for their essential services. Hence, no DCLFH® this week.
Terpenes are a class of organic compounds having the chemical formula (C5H8)n, where n is most commonly, 2 (2). There are tens of thousands of naturally occurring terpenes, mostly from plants where they are used to protect the plant from predators. Humans use them, too, for their scent, flavor, and medicinal properties. Here are four of them.
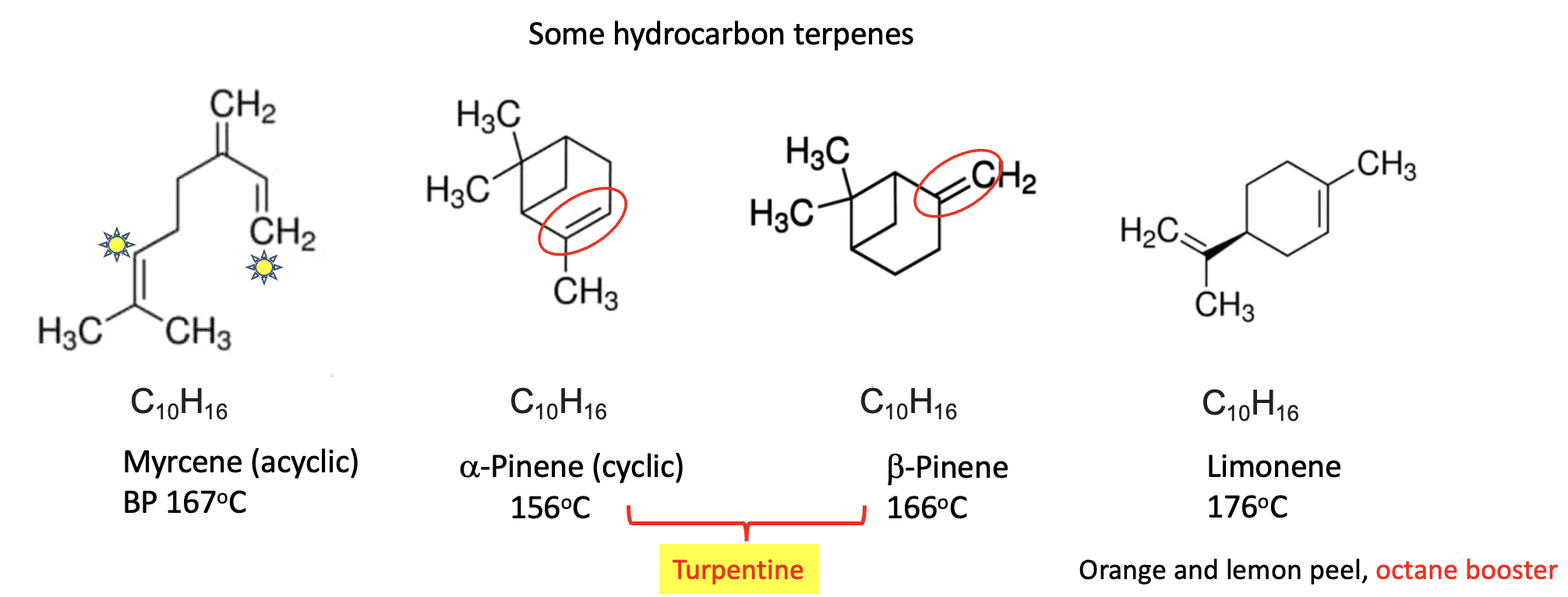
Hydrocarbon terpenes (containing only hydrogen and carbon) which makes them the most volatile members of the group. The four above are all isomers (they all have the chemical formula C10H16) and this gives them similar chemical properties, relatively low boiling points (close to 160oC) that are non-polar solvents, just like gasoline or kerosene. Myrcene is an acyclic terpene (the carbon atoms are linear rather than being in a ring). (3) Alpha and beta-pinene are isomers differing only by the position of the double bond (red oval) (4). Together they make up much of the mass of turpentine. Limonene is a major oily constituent of citrus peels. Like myrcene and the pinenes, it is volatile and flammable. Limonene has found use as an octane booster in gasoline.
Are they interchangeable?
Yes and no. All four are non-polar liquids, so they will be effective as paint removers and thinners for oil-based paints. You might be perfectly content inhaling myrcene from a joint but should probably avoid doing so with a can of turpentine from Home Depot.

All four will burn in an automobile engine, but even small changes in structures have a considerable impact on anti-knocking properties, so while limonene may help your engine run smoothly the others could screw it up. You might want to avoid this experiment, too.
One take-home message is that all four of these chemicals are found in cannabis. Along with a whole lot more. Nope, there is nothing simple about marijuana chemistry.
NOTES:
(1) In reality, it's probably neither. Compounds with cell-based anti-cancer activity are a dime-a-dozen. Very few of them actually become drugs. And if it's really a carcinogen, you'd better stop eating mangoes, basil, parsley, and guava. Among others. Of course, dose must be taken into account.
(2) Technically, a terpene contains two five-carbon units hooked together. When there are three, it is called a sesquiterpene (great Scrabble word), and four make a diterpene. Why 5-carbon units? You don't want to know.
(3) If the two carbon atoms marked with yellow stars are connected, they will form a 6-membered ring just like limonene.
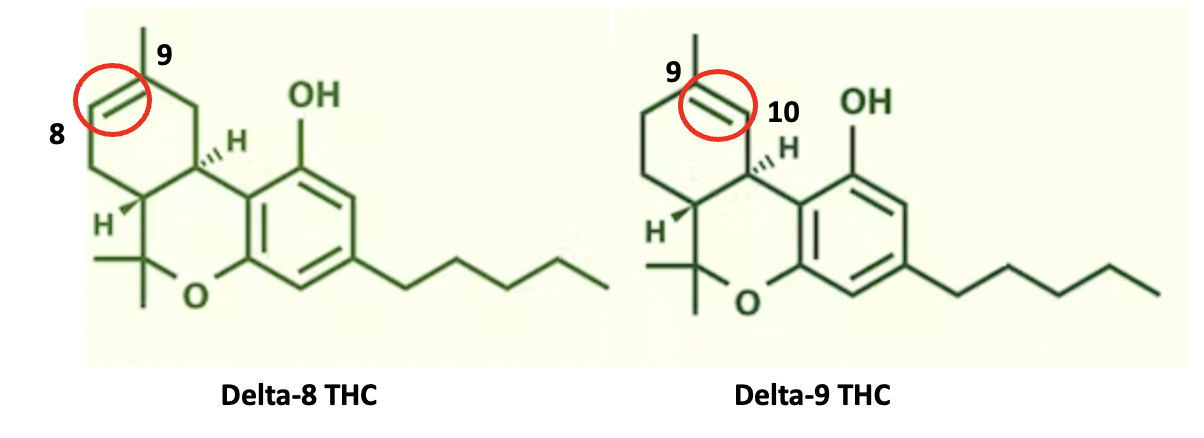
(4) Here's an example of double-bond isomers you stoners have probably heard of.