It would be impossible to guess how much money McNeil Consumer Healthcare, now a subsidiary of Johnson & Johnson, has made since it began selling Tylenol in 1955. Tylenol, a drug that I've criticized frequently (see here, here, and here) has an undeserved reputation for being safe and effective. It is neither.
Ignoring its very limited capacity to treat pain, acetaminophen, the drug in Tylenol can also be a very dangerous liver toxin at doses that are not much higher than the drug's effective dose (1). While there is an effective drug, N-acetylcysteine, to neutralize acetaminophen in the blood and prevent serious liver damage, there is nothing that can be done once the damage is done.
Irony time
Reading about JNJ-26366821, a drug that J&J has in development to "repair acetaminophen-induced liver injury in mice," was an "eyebrow-raising moment." But it's true; this story was recently reported on the BioWorld site. J&J, at least indirectly, seems to be saying: "OK, you took our drug and it screwed up your liver. Now, we're trying to figure out how to repair the damage."
Not the best optics ever.
What is JNJ-26366821?
JNJ-26366821 is a PEGylated derivative of thrombopoietin (TPO), a glycoprotein hormone; it is a major regulator of platelet formation. Let's translate this incomprehensible sentence into English using AI, which is pretty good at handling chemistry questions:
ChatGPTEnglish:
"A glycoprotein is a type of protein that contains carbohydrate chains (glycans) attached to it. These carbohydrate chains are covalently linked to specific amino acid residues on the protein surface, and they can vary in length, composition, and structure." (Nice going!)
"PEGylation is a process that involves attaching a polyethylene glycol (PEG) molecule to a protein or peptide. This modification is used in various medical applications to improve the solubility, stability, and pharmacokinetics of the protein". (Very good, indeed)
Thrombopoietin (TPO) is a hormone produced primarily by the liver and kidneys, which stimulates the production and maturation of platelets (thrombocytes) in the bone marrow. Platelets are small, disk-shaped cells that play a crucial role in blood clotting, which is important in preventing excessive bleeding following injury. (Nailed it again. Nice job, Mr. AI)
Not quite enough chemistry for a Dreaded Chemistry Lesson From Hell®. But close.
How is PEG attached to proteins? At the risk of going a little whacko on you, this shouldn't be too bad. It's standard organic chemistry (Figure 1). It's also important since there are 30 approved pegylated drugs that treat a wide variety of conditions. An especially interesting PEG drug, pegylated interferon lambda, is under development for Covid. So far, it has cut hospitalizations by half.
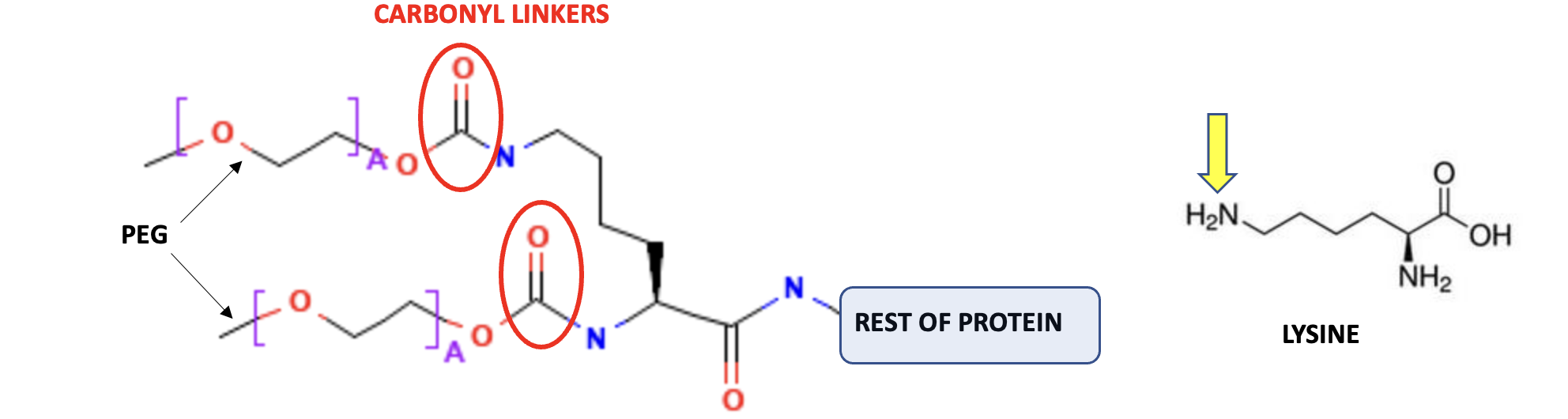
Figure 1. (Right) Representation of a PEGylated protein. Polyethylene glycol (PEG, typically with about 30 repeating ethylene glycol units, represented by A) is covalently attached to amino acid side chains (in this case lysine). Doing so gives the protein better pharmaceutical properties. (Left) The chemical structure of lysine. The yellow arrow indicates where linkage to PEG takes place. (Quiz: why is the carbonyl group needed? Nerds only)
JNJ-26366821 is a chameleon
The term "disease without a cure" can be turned around here. JNJ-26366821 is, at least so far, a cure without a disease. Scientists studying the drug are nothing if not perseverant. JNJ-26366821 is being tried for a number of conditions, although it has no approved use so far. Here are some of them:
1. Thrombocytopenia (low platelet count)
According to the Pharmaceutical Technology website, JNJ-26366821 is in Phase I trials for the treatment of thrombocytopenia. Statistically, at least, it looks good so far:
Phase I drugs for thrombocytopenia have an 85% phase transition success rate (PTSR) indication benchmark for progressing into Phase II.
2. Nuclear war
The compound has been tested in mice for protection against lethal radiation (make sure you bring this up at the next PETA meeting) and was 30-90% effective in prolonging their lives. According to a Nature paper:
These results support the potential use of JNJ-26366821 as a medical countermeasure for treatment of acute TBI [total body irradiation] exposure in case of a radiological/nuclear event when administered from 4 to 24 h post-TBI.
If this doesn't help you sleep well at night I don't know what will.
3. Thrombocytopenia resulting from acute myeloid leukemia (AML)
AML is a cancer of the bone marrow that involves the overproduction of immature myeloid cells. These cells will not produce red blood cells and platelets. The problem here is that any drug that stimulates the production of myeloid cells can also accelerate the cancer. However, a paper in the journal Leukemia and Lymphoma reported that JNJ-26366821 was able to stimulate platelet formation without exacerbating the AML.
4. Acetaminophen-induced liver damage
There's little information on the preclinical studies of JNJ-26366821 in repairing liver damage in mice. Make that very little. The following is from the BioWorld website:
JNJ-26366821 repairs acetaminophen-induced liver injury in mice
Acetaminophen (APAP) is a very common nonprescription analgesic, harmless at low doses, that can cause acute liver injury and even death from acute liver failure when overdosed. The temporal course of acetaminophen overdose-induced liver injury (AILI) can be depicted in two stages – injury and recovery.
Then this:
To read the full story, subscribe or sign in.
In order to sign in one needs to subscribe. No problem, right?
Wrong.

Well, that's not terribly affordable. Perhaps they have cheaper plans.
No.
How about a single reprint?
Please.

Hardly a screaming deal either. So, I guess if, for some reason, you want to know more about how those mice experiments you're on your own. Should you be so inclined please send us a copy. It's tax-deductible!
NOTE:
1) The maximum FDA-recommended dose for Tylenol is 3,000 mg per day. The effective dose is...I don't know because it's not effective for most types of pain. The minimum toxic dose is as little as 7,500 mg.




