While we all enjoy cleaner air and less airborne particulate matter is always better than more, public health policies must be based on demonstrated human health benefits.
Reducing tumors in laboratory mice is not one of them
Because of the journal’s (Nature) publication paywall, we are limited to the study’s abstract. Chief among the missing detail is any description of the PM2.5 exposures involved, such as duration, frequency, concentration, or composition, or whether the particles were collected from ambient sampling filters or produced in a laboratory. The abstract only says that “4 within-country cohorts” were involved. In a related Chinese toxicology study, pulmonary injuries and lung cancer progression were observed in mice exposed to 170 μg/m3 PM2.5 for six hours daily for up to 3 months. This concentration is about 16 times the current US average, and the delay between exposure and diagnosis (latency) was not addressed.
- What evidence indicates that air pollution might cause cancer?
- Differences in rates by location must account for the timing of exposures.
- Differences over time must consider locations, including indoors. Acute effects are not relevant.
- Differences by occupation must consider the duration of employment.
- Differences in smoking and its correlates must always be considered as confounders.
- Are particulates the most likely airborne agent?
- Solid and radioactive (radon) materials may accumulate in the respiratory system.
- Which particles are carcinogenic in toxicology studies?
- Metals (Cr, Ni, V, Cd, As), soot, polycyclic aromatic hydrocarbons; pollutants not routinely monitored in the US.
Each of these topics has been considered in the literature, but no published study has considered all of them.
I searched the NIH PubMed database for epidemiologic lung cancer studies that included progressively more specific topics and combinations. Potential carcinogens, including tobacco, radon, asbestos, heavy metals, and second-hand smoke (SHS), were mentioned most often; individual air pollutants (PM2.5, NO2, O3, polycyclic aromatic hydrocarbons (PAHs), soot, ultrafine particles, benzo(alpha)pyrene (BaP), black carbon) much less so. Thousands of publications have considered one factor at a given time; the number of citations with more than one pollutant, cumulative exposures, or latency is in single digits.
A recent large-scale epidemiology study of US cancer incidence and PM2.5 represented improvements but had significant design problems.
- 8.7 million cases were diagnosed from 1992 -2016, but only ten states were represented.
- Only one pollutant (outdoor PM2.5) was considered.
- Moving-average PM2.5 levels over 15 years were considered, but cumulative exposures were not.
- Data on important behavioral and socioeconomic factors, including smoking, were only available for counties, not individual patients.
- Five of the 26 specific cancers considered (oral, colon, rectal, lung, skin) were significantly associated with PM2.5, of which rectal and lung cancers were the strongest. It is difficult to postulate a common causal mechanism for such disparate sites.
- For smoking, the PM2.5-lung cancer dose-response function leveled off rather than increasing exponentially.
This study illustrates the futility of having many cases with insufficient information on each, sacrificing accuracy for precision. Its appropriate conclusion is that some cancer patients may have lived with higher PM2.5 levels but did not necessarily incur cancer because of them.
A less problematic lung cancer cohort study involved 20,625 French national gas and energy company (EDF) employees recruited in 1989. 349 new lung cancers were recorded through 2015. Features of this study include:
- Outcomes included incidence of any cancer and lung cancer.
- Detailed individual data on 15 potentially confounding variables, including passive smoking, occupation, and traffic exposures.
- Cumulative exposures and latency were considered.
- Exposures to black carbon (BC), PM2.5, and NO2 at individual residences were determined, but indoor measurements were not made.
- Ambient BC levels were similar to estimated US levels; PM2.5 and NO2 levels were higher.
The relative risk of lung cancer incidence associated with BC was about 2.0. About 186 of the 3711 cancers at any site were associated with BC, including 84 lung cancers. There were significant cumulative risks of cancer-related to BC after adjustment for PM2.5 or NO2, neither of which was significant on its own. An important finding not discussed by the authors is that the median exposure levels were nearly identical for cancer patients and other cohort members, such that the findings of pollution-associated risks depended on the effect of non-pollution factors such as smoking. This study has not been cited elsewhere, by contrast with US studies used to support PM2.5 regulations.
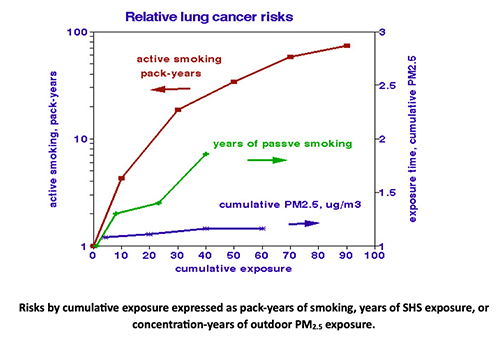 The figure compares dose-response associations of lung cancer with cumulative exposures to smoking and PM2.5. Risks from active smoking (left-hand logarithmic scale) increased exponentially with the total amount smoked. Risks from passive smoking (right-hand linear scale) increase proportionately over time. Lung cancer risks associated with PM2.5 are essentially independent of exposure time, indicating that the PM2.5 risk is a characteristic of those exposed rather than the amount they inhaled. At 2 packs a day over 20 years, 40 pack-years, smoking is roughly 20-fold riskier than PM2.5. If 5% of the smoking effect were mistakenly attributed to air pollution, the “risk” from air pollutants doubles.
The figure compares dose-response associations of lung cancer with cumulative exposures to smoking and PM2.5. Risks from active smoking (left-hand logarithmic scale) increased exponentially with the total amount smoked. Risks from passive smoking (right-hand linear scale) increase proportionately over time. Lung cancer risks associated with PM2.5 are essentially independent of exposure time, indicating that the PM2.5 risk is a characteristic of those exposed rather than the amount they inhaled. At 2 packs a day over 20 years, 40 pack-years, smoking is roughly 20-fold riskier than PM2.5. If 5% of the smoking effect were mistakenly attributed to air pollution, the “risk” from air pollutants doubles.
Lessons from this review include:
- Only a few published lung cancer studies consider all relevant factors.
- Relevant air pollution exposures include a range of pollutants, indoors and outdoors, lagged to account for latency, and summed over the latency period.
- The carcinogenicity of ambient PM2.5 has not been determined independently of these other factors.
- Black carbon should be routinely monitored in the US.
Toxicology studies describe what could happen under controlled laboratory conditions. Epidemiology studies tell us what actually happened in our real world.



