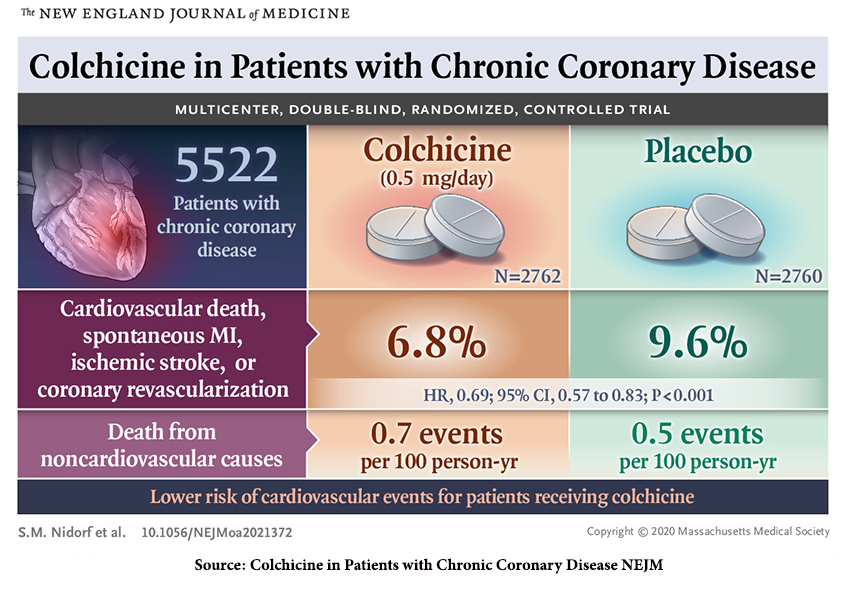
The study by Agepha Pharma used by the FDA in its approval of this new indication for use ultimately involved 5478 patients between the ages of 35 and 82 with evidence of coronary artery disease upon imaging [1] and had been clinically stable for six months before enrollment. A larger cohort of 6528 patients was initially included and underwent a one-month open-label use of colchicine. Those who were not compliant, clinically unstable, or had “unacceptable” side effects from the colchicine were removed. [2] Patients were randomized to treatment with 0.5 mg of colchicine daily or placebo.
85% of patients had a history of an acute coronary event, severe chest pain, or myocardial infarction, two-thirds of which were more than two years previously. Mean age was 66, predominantly a male population at 84%, with 18% having diabetes and 12% smoking. 83% had undergone operative and percutaneous coronary revascularization. All took an antiplatelet or anticoagulant and lipid-lowering agent, usually a statin. They were well-matched with the placebo group.
We might best describe the study group as having well-managed chronic coronary artery disease, who were compliant with additional daily medication and, to a large degree, had no adverse effects from colchicine. Despite that, 10% in each study arm discontinued the drug or placebo over the course of a 26-month follow-up.
Results
- The impact of colchicine was most significant for myocardial infarction, followed by coronary revascularization - coronary artery bypass grafting or stenting
- Colchicine appeared to have no statistically significant impact on ischemic stroke or cardiovascular deaths.
- As might be anticipated, there was a lower, but statistically insignificant, reduction in gout, for which colchicine has been a classic treatment, in the treatment arm of this study.
- Colchicine had side effects that prompted 12% of the total cohort to drop out of the study.
- The study’s primary limitations were the few women enrolled, the lack of laboratory data on lipid levels, and, more importantly, in evaluating C-reactive protein, an important biomarker of systemic inflammation.
Based on those findings, the FDA approved colchicine for the following indications:
“Lodoco is indicated to reduce the risk of myocardial infarction (MI), stroke, coronary revascularization, and cardiovascular death in adult patients with established atherosclerotic disease or with multiple risk factors for cardiovascular disease.”
You will note a bit of human judgment in this labeling since the impact of Lodoco, the brand name of the approved colchicine, was tested on individuals with established atherosclerotic disease rather than multiple risk factors, and the reduction in cardiovascular deaths was not statistically significant.
Why colchicine?
While LDL cholesterol gets all the attention, low-grade systemic inflammation, as measured by increasing low levels of C-reactive protein (CRP), is a “powerful determinant of recurrent cardiovascular (CV) events, CV death,” independent of the level of LDLs. Colchicine reduces inflammation by interfering with neutrophils, an essential cell in inflammation, reducing their ability to travel and stick to the inner lining of blood vessels. This is a new tool in the ongoing attempt to reduce cardiovascular disease globally.
An economic moment.
An early signal of the impact of colchicine on cardiovascular disease was identified in retrospective studies of patients with gout and Familial Mediterranean Fever who had a lower incidence of coronary disease than their untreated contemporaries. Agepha Pharma, the company just winning approval for this new use of colchicine, spent the time and money over two clinical trials, Lodoco 1 and 2 (the one used to win FDA approval), to demonstrate a clinical benefit.
Colchicine has been around for ages, literally. It was approved for use in 1961 by the FDA for gout but, because of the regulatory statutes, or a lack thereof, did not require a safety evaluation. That situation changed in 2007 with the FDA Unapproved Drugs Initiative, which was intended “to reduce the number of drugs available on the market that lack FDA-approved New Drug Applications (NDAs) or Abbreviated New Drug Applications (ANDAs).” An unintended consequence was that “manufacturers that took previously unapproved drugs through the FDA approval process” could gain exclusivity.
As reported by Fierce Pharma, one pharmaceutical company, URL Pharma, took the time and money to conduct the necessary trial. When the FDA approved their branded version of colchicine (Colcrys) in the treatment of gout, the cost of a dose of colchicine rose from 9 cents to $5. This graphic from a JAMA article entitled Changes in Prescription Drug and Health Care Use Over 9 Years After the Large Drug Price Increase for Colchicine is an excellent demonstration of the relationship between supply and demand. Colcrys was approved in late 2009, just before that rise in price.
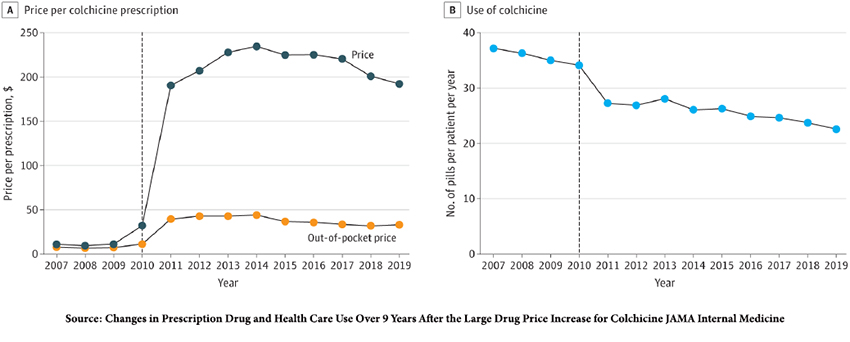
“The study’s findings suggest that the large and sharp increase in colchicine prices was associated with a sustained decrease in colchicine use, increased use of other medications for gout, and increased clinical encounters for gout, consistent with poorer disease control.”
Of course, medical economists have considered both colchicine's additional cost of colchicine and life enhancement, as reported in the Canadian Journal of Cardiology Open. If colchicine is priced at $9 per dose, it would reduce the overall cost of standard care for coronary artery disease by roughly $5,500 [3] and add 1.5 quality months of life. Colcrys is currently priced at about $8 a dose; Lodoco’s price has yet to be announced. [4]
[1] Imaging could include conventional cardiac catheterization, CT coronary angiography, or a coronary-artery calcium score of >400 (increased risk of myocardial infarction).
[2] The most common reason for exclusion was gastrointestinal upset.
[3] Primarily by reducing additional hospitalization, imaging and medical interventions.
[4] Subsequent to our initial posting, we have been told that for those with insurance, the cost will be about $30 for a 3-month supply. For those without insurance, the cost rises to $99/month with an "available patient assistance program."
Source: Colchicine in Patients with Chronic Coronary Disease NEJM DOI: 10.1056/NEJMoa2021372