
Wanna make some easy money? If so, you'd be wise to ignore this article. But if you want to be enriched in knowledge, feast away. But don't try this at home.
Recently, some nut wrote to me wondering why we haven't had a Dreaded Chemistry Lesson From Hell® recently. Well, here's the lesson, but my public relations skills are obviously not well honed. Otherwise, I would not refer to a long-time friend of ACSH (and a generous donor) as a nut. It would seem that my fundraising skills are no better. Fortunately, this fine gentleman has a sense of humor and will understand that random insults can escape at any given time, with or without the slightest provocation. Let's call this condition "Chemical Tourette's." He'll understand.
Reasons to and to not purify silver
The charts below show the rise in gold and silver prices over the past 5 years. They've both roughly doubled.
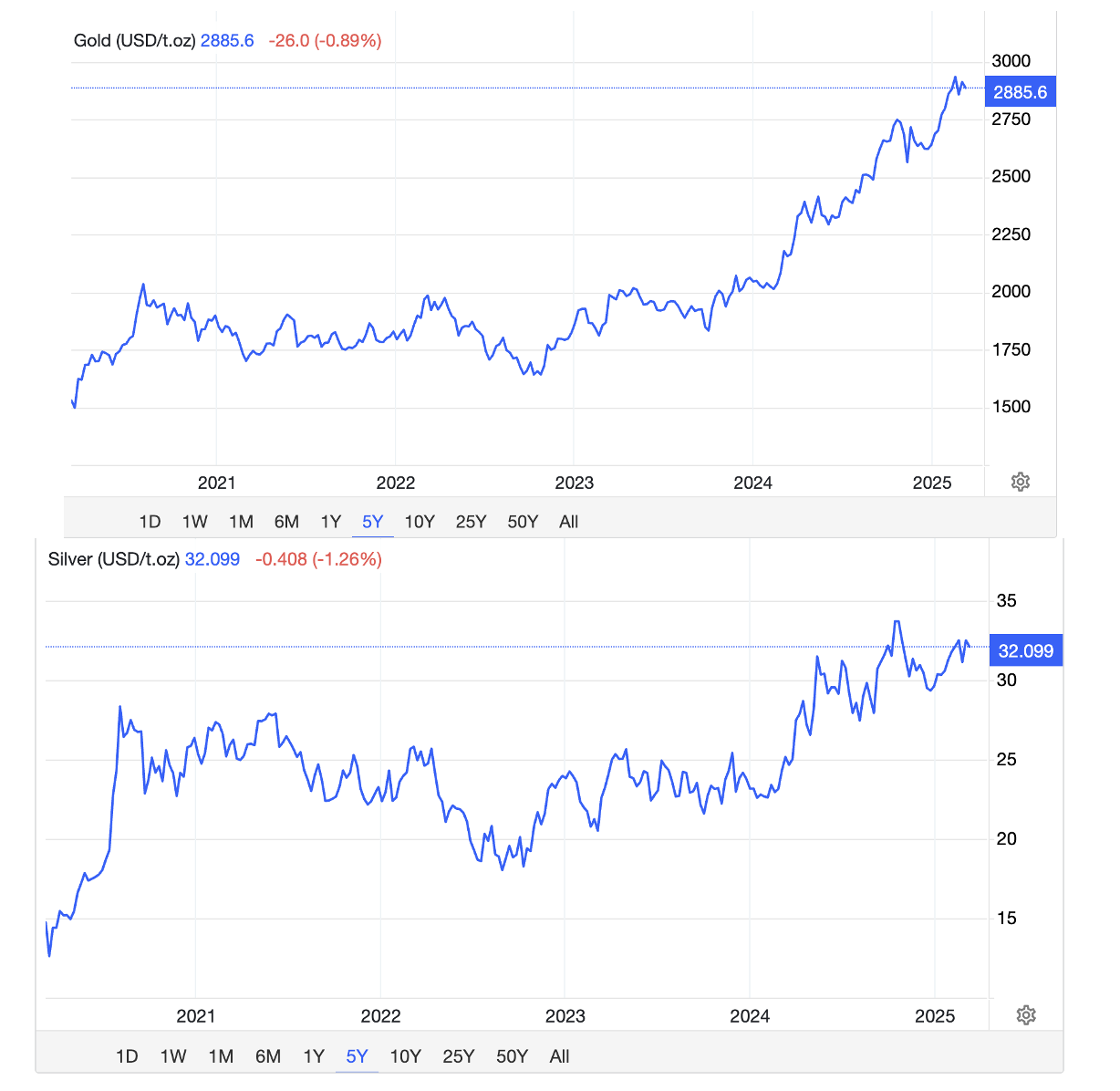
Figure 1. The prices of gold and silver over the past 5 years. The price of gold increases during times of uncertainty (no shortage of this nowadays). Silver often tags along. Source: Trading Economics
Suppose you need to buy an egg and are looking for fundage to do so. A home egg-quity loan isn't wise; the rates suck. Perhaps you have a stash of old silver coins (1) in the attic? You could sell them on ebay, where a pre-1965 quarter will fetch you $5.75 ("junk silver" coins–those without numismatic value– have value because of the silver content in the coin. But silver coins are only 90% silver (the rest being copper) and this does not meet your high standards. A little chemistry will set you free! And you know what that means...another marginally popular Dreaded Chemistry Lesson From Hell®! Shall we?
It's been a while, but our hosts of the DCLFH , Steve and Irving, don't really have anywhere better to be, so when I asked them to present some chemistry on silver they crankily obliged.

Steve (left) and Irving, all blinged out for the occasion, are puzzled by the presence of Vanilla Ice, who undoubtedly melted–some time ago.
How to get pure silver from old coins
The chemistry is pretty easy, but doing it without killing yourself–not so much. This is because the first step involves the use of concentrated nitric acid, which will eat through pretty much everything, including your epidermis, dermis, and all the other dermises it encounters.
STEP 1 - Dissolution
Pour some about 25 mL of concentrated nitric acid into a beaker. Toss in a silver/copper quarter. You'd better do this in a fume hood because a whole lot of nasty-ass brown gas, nitrogen dioxide will come pouring out of the beaker because it goes batshit crazy. This can be seen from a YouTube video where a penny is eaten alive.
Here's the reaction:
3 Cu+8 HNO3→3 Cu(NO3)2+2 NO+4 H2O
Copper plus nitric acid ---> Copper (II) nitrate + Nitrous Oxide + Water
The reaction with silver looks much the same (2)
Ag+2 HNO3→AgNO3+NO2+H2O
STEP 2 - Precipitation
Assuming you haven't died from Step 1, next you add a whole bunch of water to dilute the hellish brew. Then chuck in some salt and the purification process begins. How? It's all about solubility of the two chloride salts:
Copper chloride: 6.2 mg/100 mL of water @25oC
Silver chloride: 0.019 g/100 mL of water @25oC
Once the sodium chloride is added, the copper chloride stays dissolved while the much less soluble (>300-times) silver chloride will precipitate as a while solid in the bottom of the beaker. Collect this by filtration and the copper and silver will be separated
STEP 3 - Conversion
OK, now you have a bunch of silver chloride on your hands (hopefully not ON your hands). It's quite easy to convert this to silver, possibly even without killing yourself. You need concentrated ammonia (aka ammonium hydroxide), something you do not want to get a snootful of. It feels a bit like poking a soldering iron in both eyes.
Here's the cool part. Although silver chloride is insoluble in water it is very soluble in ammonia because of another reaction:
AgCl (solid)+ 2 NH3(aqueous) → [Ag(NH3)2]+Cl−(aqueous)
Solid silver chloride (insol) + aqueous ammonia ---> Silver ammonium chloride complex solution ((aqueous)
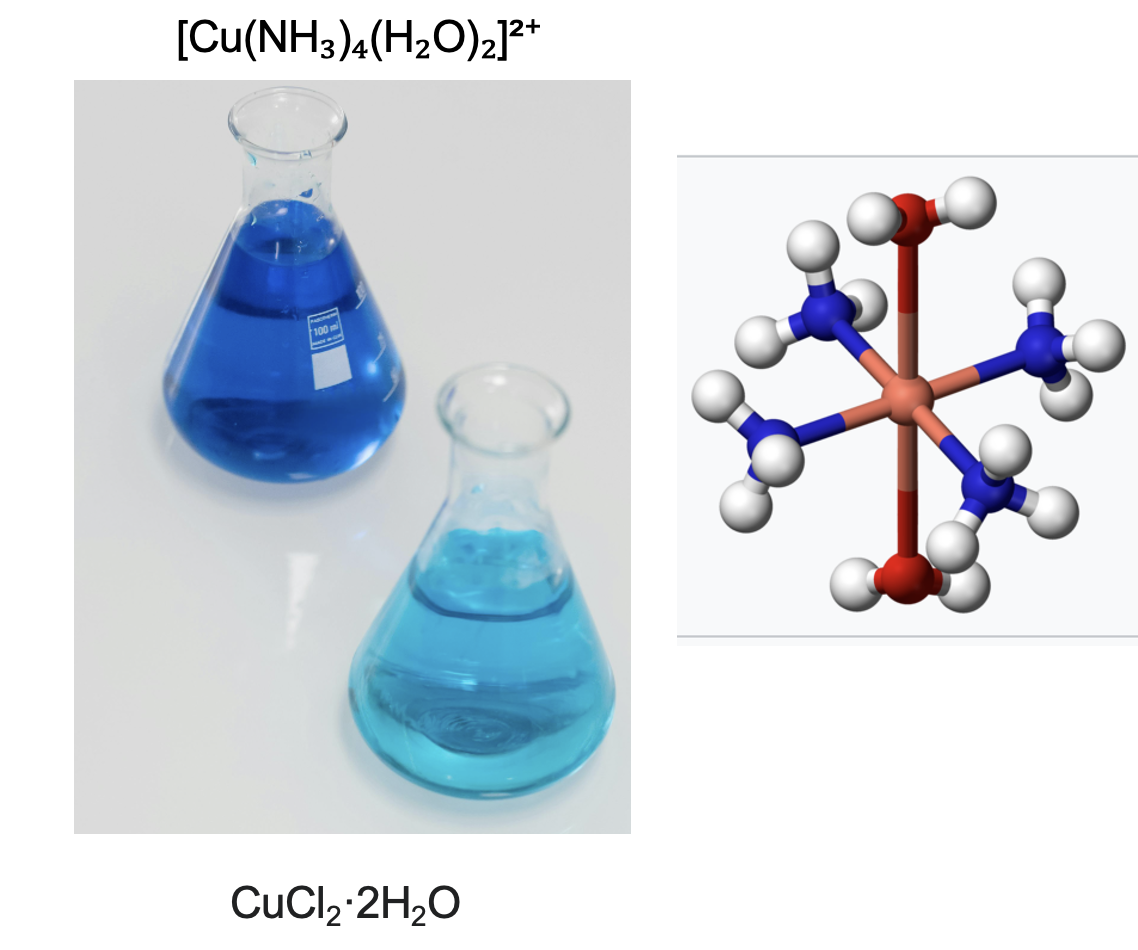
(Left) Copper II chloride turns from light blue to dark blue when ammonia is added. (Right) The 3D structure of the complex contains a central copper ion (red) bound to four ammonia molecules (blue and white spheres) and two molecules of water (red and white spheres). Credit: Pexels. The silver ammonia complex is a colorless solution, looking like water.
STEP 4 - Reduction
To recover the pure silver the silver ammonium complex Ag(I) needs to be reduced to Ago. There are a whole bunch of reagents that can accomplish, including glucose/sodium hydroxide or formaldehyde, but the best method is using sodium dithionite, which is quite safe. This finishes your lab session, probably for good:
2[Ag(NH3)2]+ +Na2S2O4+ H2O → 2 Ag(solid)+2NH4++2NH3+2 SO3-2+2 Na+
Silver ammonium complex + Sodium dithionite --> Silver metal + A bunch of soluble crap.
Then, filter it and you have pure silver powder.

Silver metal powder. Boring but kinda valuable.
Was it worth it?
Of course not. (Just like reading this article.) One pre-1965, 90% silver quarter weighs 6.25g. If you go through the horror show above and assume 100% yield on every step you will end up with 5.625 g of pure silver. At today's price–$1.04 per gram–you will have made $5.85 worth of pure silver. If you had sold the damn quarter to a coin dealer you'd get $5.75. That's almost as big a waste of time as this lesson.
Plus, in order to earn that extra 10 cents you'll need to figure in the cost of the ambulance ride, ER costs to (maybe) save your sight, and 20/600 Coke bottle lenses to be able to see a Great Dane five feet away.
He shoulda sold the quarter.
Note:
(1) Once 1965 came along virtually all the older silver coins (dime, quarter, half dollar, dollar) were hoarded for their silver value. Once in a while you'd stumble across one. By 1970 you have just as good a chance of finding a Studebaker in your pocket.



