Here's a chemical that you don't want to screw around with: sulfuric acid. The only thing good about it is that it is not volatile. The only thing bad about it is everything else.
And it's pouring out of the Kilauea volcano as we speak. Sort of.
Many chemists hate working with concentrated sulfuric acid. “Add acid to water” is good advice for all acids, but sulfuric acid is the one where you’d better pay attention. If you add even a small amount of water to concentrated sulfuric acid, the first drops can flash-heat on contact, sometimes boiling instantly and “spitting” hot acid out of the container, often in the general direction of your face. This is something you can do without.
Another, safer way to demonstrate the same thing is by placing both a half-full beaker of water and another half-filled with concentrated sulfuric acid in a closed system and letting it sit for a week or two. The water level will steadily drop as it evaporates and is scavenged by the sulfuric acid, which becomes more dilute (Figure 1). Perhaps you’ve seen this experiment.
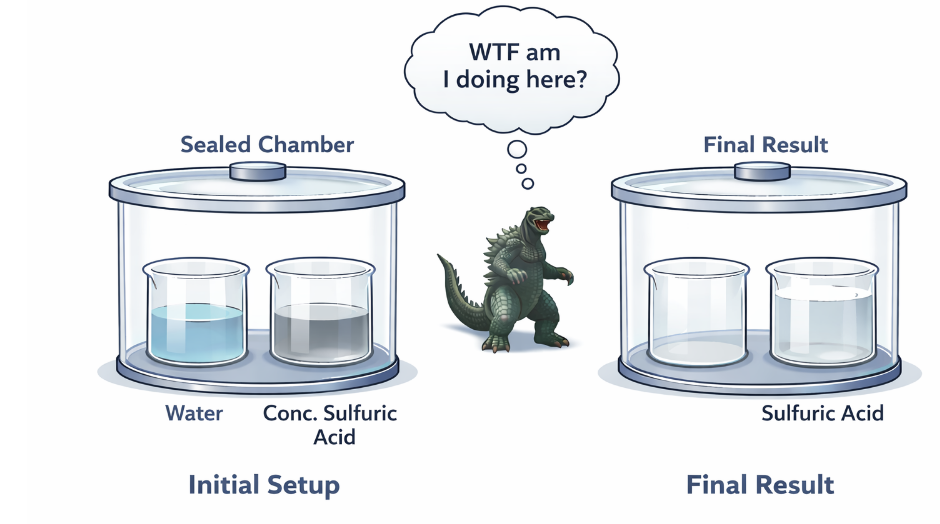
Figure 1. A depiction of the sulfuric acid water experiment. A beaker of concentrated sulfuric acid and a beaker of water are placed in a closed system, often a desiccator. Neither Godzilla nor I has the wildest idea of why he's there.
Sulfuric acid ruins stuff
- Sulfuric acid has a sweet tooth. This nightmarish video shows what happens when it reacts with sugar.
- The video of what it does to a sock is hilarious.
- A chicken leg doesn't have a leg to stand on.
And, some lunatic wanted to see how big the explosion is when a chunk of sodium is dropped into a bowl of the stuff – a terrible idea by any measure. It doesn't disappoint.
Sulfuric acid also loves all types of clothing. No matter how careful you are, if you're using the stuff in the lab, your clothing is likely to end up with small holes. Yet, this is less of a problem than one might think.
It is no secret that we chemists are not known for our sartorial splendor. Not only doesn't it matter that we might walk around with holes in our clothing, but some of us actually think this looks pretty good. Polyester plaid shirts with holes and a striped tie were an especially badass look for a while. (I did, however, take issue with pants coming to a rest roughly midway between the ankles and knees.)

This is closer to reality than you might expect.
Volcano chemistry - What are you breathing?
Volcanoes put out ginormous amounts of a putrid-smelling gas called sulfur dioxide (SO₂). Yet the real danger in breathing volcanic air is not SO₂ — which is bad enough — but, as should not be surprising, sulfuric acid. But here’s a conundrum:
Volcanoes expel very little sulfuric acid. Yet, on some days there is so much of it in the air that sections of the park are closed. Where did it come from? Sulfur dioxide doesn’t turn into sulfuric acid just because it encounters water.
The answer? Mother Nature is needed not once but twice — it requires both water and sunlight.
Step 1: A stinky gas turns into a weak acid
Sulfur dioxide reacts with water, forming sulfurous acid (H2SO3).
SO2 +H2O → H2SO3
Even you art history majors can tell the difference. Where does the other oxygen come from? That's where sunlight and air come in.
Step 2: A weak acid turns into a very strong one.
H2SO3 + O2 [catalyzed by sunlight] ------> H2SO4
Sunlight-driven atmospheric chemistry oxidizes sulfur from (IV) to (VI), converting sulfurous acid (weak) into sulfuric acid. The reasons behind this are beyond the scope of this article.
This is why if you are near a volcano, you will feel a burning sensation–minuscule particles of sulfuric acid in your eyes, nose, and lungs (2), but there is a different kind of burning. It comes from molten lava.
How hot is molten lava? And how do you measure the temperature?
Molten lava is crazy hot. Just look at the latest videos of it incinerating everything in its path. The lava pouring from Kilauea is 2,140 ºF, not too different from Bikram yoga. That's plenty hot. Just for laughs, here are the melting points of some common metals (in ºF). Lava won't melt every metal, but it takes care of its share.
- Gold - 1948
- Silver - 1763
- Lead - 621
- Iron - 2800
- Titanium - 3034
- Aluminum - 1221
And glass - ~ 2700
So, while you could theoretically take a glass thermometer, stroll over and stick it in the molten lava, this is not recommended. But something pretty close to this is done. A thermocouple consists of twisted pieces of two different metals. When heated, this will generate an electric current that can be measured and will be indicative of the temperature. I volunteer you for this.

Lunatic measuring lava temperature in Hawaii, 1971. Photo: Hilo.hawaii.edu
Far better is the use of color, which is done from a distance. The color of the lava is related to its temperature through a godforsaken entity known as the Stefan–Boltzmann law. On the surface, it appears simple enough:
The Stefan-Boltzmann law. Indecipherable jibberish.
But it allows one to measure temperature from the radiation emitted by hot objects. At lava temperatures, some of that radiation is visible, which is why the lava actually glows. There are actually people out there who understand this stuff. I am not one of them. If you ask me to explain this, I will just hide under the bed. Source: Wikipedia
That's about it. Ran out of gas. Aloha.

Don Ho agrees. Image: Wikimedia Commons




