
When is the last time you thought about boron? Probably never. I mean, talk about a snoozer. This unfortunate element gets absolutely no respect from its neighbors on the periodic table.
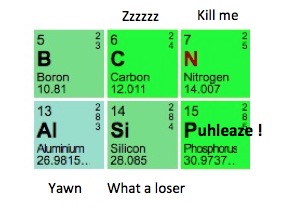
Boron being disrespected by its periodic neighbors
But, if boron were really that boring, I wouldn't take the time to write about it. When you learn a little about the old boy, he's way more interesting than you might think.
If you ask 100 people on the street in New York to tell you one fact about boron, the following will happen:
- 75 will be looking at their iPhones so that they won't answer.
- 11 will view you as an escaped mental patient and ignore you
- Seven will hit you
- Five will scream stuff about Jesus in your face
- Two will throw up on you
- One will say boric acid

Boric acid (B3HO3) is pretty boring, but it sure has a lot of uses. Here are a few:
- Roach killer
- Antiseptic
- Making Silly Putty
- Athlete's foot
- To keep nuclear power plants from melting down.
(Go ahead. Try to find anything else that does all of this.)
Elemental boron is not found on earth; it exists solely in minerals. Some of them are very cool (below). But it is possible to purify it to give the element, a brittle gray metal.

Elemental boron (yawn). Photo: Wikipedia
But the minerals in which elemental boron is found are quite a bit more interesting (1). 
Photos: ChinaMet.com, Wikipedia, csmsgeologypost.blogspot.com, The Earth Story
Chemists will (almost) never use elemental boron or its minerals. But there are commonly used chemical reagents (2) that are found in most labs. But, many are 50 shades of nasty. For example, when boron is bound to hydrogen, you get a class of compounds called boranes (3). You have to be a bit careful with them because they are not especially fond of air or water. For example, below is diborane, the simplest member of the class.
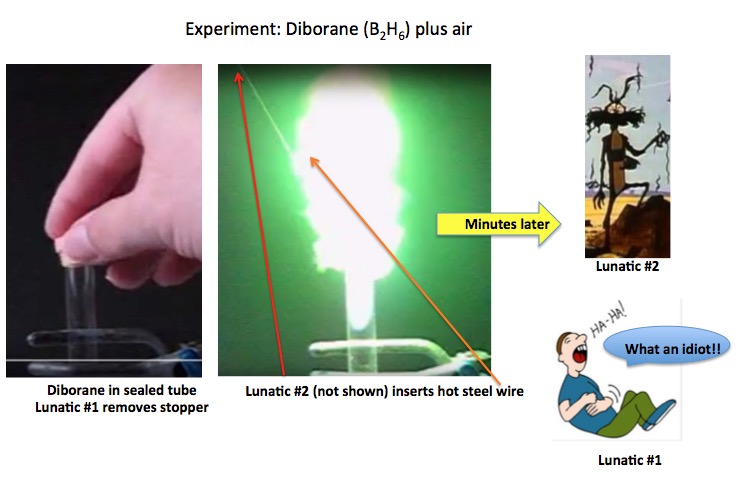
Photos: YouTube, Shutterstock
If this seems bad, it is a Hershey's Kiss compared to another member of this class. It is called pentaborane, and, not surprisingly, it is on Derek Lowe's famous "Things I Won't Work With" list.
I came across the book [Max Gergel’s extraordinary memoir “Excuse Me Sir, Would You Like to Buy a Kilo of Isopropyl Bromide?”] in Duke’s chemistry library in 1984, a few years after its publication, and read it straight through with my hair gradually rising upwards.
Derek Lowe, In the Pipeline, describing his reaction when he read about pentaborane in Gergel's book.

Derek has plenty of hair to rise upwards. Must have been quite a sight.
And who can blame him? The stuff is pure evil.
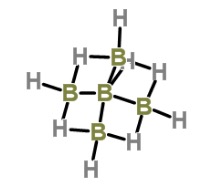
Pentaborane- BBBBBBad to the bone. Image: ChemSpider
During the process of detoxifying old canisters filled with pentaborane gas at an industrial site in Virginia, two workers were seriously injured and one died... [Th]e most acutely exposed worker began having convulsions 4 min after dermal contact, and 4 min later had an opisthotonic spasm and went limp. He had erythema and marked congestion of the conjunctiva and oral mucous membranes. An electroencephalogram (EEG) conducted 6 h after the exposure revealed no electrical activity, and the worker died 8 days later. Autopsy revealed severe necrotizing pneumonia, fatty changes with centrilobular degeneration in the liver, brain degeneration, and lack of mature spermatozoa in the testicles.
National Academic Press, "Pentaborane: Acute Exposure Guideline Levels."
Good luck topping that.
Perhaps the most interesting boron-containing chemical of all is one that nature made - boromycin - the first ever natural product to contain a boron atom (4), and one of the strangest structures you'll ever see. Boromycin is an antibiotic that was first discovered in 1967, but if you were an organic chemist in 1966 and even mentioned that some weird dude like this existed, you'd be carted off in a boron-free ambulance.
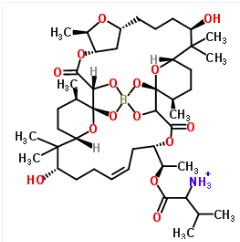
Boromycin - A "freak" of nature. Image: ChemSpider.
So, remember - boron is not boring.
Chemistry lesson is over. For both of you who read this, enjoy the lab.
Notes:
(1) Boron is colorless. Colors found in boron-containing minerals are due to impurities, usually other metals.
(2) Reagents are chemicals of all types that are used to convert one chemical compound into another. They can be gasses, metals, liquids, or solids.
(3) When I was in grad school, one of the groups specialized in borane chemistry. Since boranes cannot be exposed to air, they are kept in a complex apparatus, which allows a chemist to move the liquid into reaction vessels to do God-knows-what with it. The borane apparatus and contents are always kept under vacuum, which means that vacuum pumps are constantly running. The pumps use mercury to get a better vacuum. Everyone in the lab went bald from the mercury vapors.

(4) There are a few more. Very few.



