
I recently wrote about hexanitrogen, perhaps the world's most perfect rocket fuel, and a seriously bizarre molecule that decomposes like a madman, giving off only nitrogen gas. Zero pollution.
Now, chemists at SUNY Albany have come up with another doozie: manganese diboride (MnB2) [1]. I'm guessing that about 0% of organic chemists have heard of such a beast, let alone used it. Nonetheless, a study about its possible utility as a rocket propellant has been published in the Journal of the American Chemical Society, the most impactful chemistry publication.
Did I hear the word chemistry?? Seasoned readers know what that means...
Yes, in case you haven't already suffered enough, let's make things even worse by adding a Dreaded Chemistry Lesson from Hell®!

Steve (left) and Irving, currently waiting in a nine-hour line for admission to the Burning Man orgy tent, protest their return to host the Dreaded Chemistry Lesson from Hell®!
First, here is a list of elements that, given my nearly-nonexistent knowledge base, are least likely to be used in rocket fuel:
- Manganese
- Boron
- All the rest of them
And here are some facts about the chemical, according to Wikipedia, so they may or may not be correct. But they will give you an idea of how strange the stuff is. They may or may not be useful on Trivia Night.
- Manganese diboride is an inorganic compound of manganese and boron with the formula MnB2. Duh.
- Its superconducting mechanism is primarily described by BCS theory (named after Bardeen, Cooper, and Schrieffer, the inventors). Most normal humans have never heard of it—maybe even Bardeen, Cooper, and Schrieffer.
- Here is a simple equation that succinctly describes BCS theory. It should be self-explanatory.
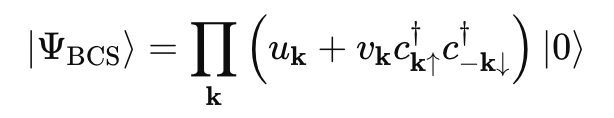
- As if this wasn't already obvious.
If you haven't offed yourself by now, here's some more information that you may or may not need:
- "The corresponding London penetration depths are 33.6 nm and 47.8 nm. This implies that the Ginzburg-Landau parameters are 0.66±0.02 and 3.68, respectively." Now you can sleep well.
- And people wonder why only mutants understand quantum mechanics.
The Albany group discovery
Here's a YouTube video that nicely explains the potential benefits of the discovery.
And here's a summary of the discovery:
- MnB₂ releases about 20% more energy per unit weight and is roughly 2.5 times as energy-dense as aluminum powder [2], the standard metal fuel in today’s solid rocket boosters.
- This means rockets could carry less fuel for the same performance, freeing up precious space and reducing overall weight—both critical limitations in spaceflight.
- For a powerful explosive, MnB₂ is remarkably stable: it only combusts when ignited with another fuel, such as kerosene
- That’s significant because it combines some of the energetic density of hydrogen with the easy handling of a solid fuel.
- If scalable [3], this could revolutionize solid rocket propulsion.
Let's end with a great quote from Michael Yeung, Assistant Professor of Chemistry at SUNY Albany, who is the lead author:
I distinctly remember the first time [as a grad student] I made a compound related to manganese diboride... There I was, holding this new material that was supposed to be super hard. Instead, it started to get hot and changed into a pretty orange color. I thought, Why is it orange? Why is it glowing? It shouldn't be glowing! That's when I realized how energetic boron compounds can be. I put a pin in it to explore in the future, and that's exactly what we are working on now.
That's science for you. Something that seems unimportant or trivial can later become just the opposite. Brains + Luck = inventions.
NOTES:
[1] The compound was first discovered in 1953. In case that makes any difference.
[2] Aluminum must be oxidized to release its energy. Ammonium perchlorate is the commonly used oxidizing agent.
[3] Scalability may be the most important issue. Here's how the authors made the stuff: "Stoichiometric powders of manganese and boron were pressed into pellets and annealed at 1000 °C; this provides an interconnected electrical connection for arc melting and ensures that the resulting pellet will be evenly heated in a rapid manner. Next, the samples were arc melted for no longer than 1 min to limit any potential decomposition, and the copper hearth was kept chilled at 9 °C to rapidly cool the molten ingot and quench the high-temperature AlB₂-type phase." Not suitable for your kitchen.



