This could be huge. Seriously.
Antibe Therapeutics, a Toronto-based pharmaceutical company, is evaluating an experimental drug in Phase II clinical trials that could be a much safer (and possibly more effective) option for the treatment of chronic pain (1) and inflammation than anything we have now. And, although it is still too early to celebrate, the data so far isn't simply good. It's great. More on that later.
It has been more than 120 years since heroin and aspirin were invented and not much has changed. The mainstays of pain relief are still non-steroidal anti-inflammatory drugs (NSAIDs) and opioids. Not great progress. In fact, one could argue that we have regressed rather than progressed since then. At least you could get powerful drugs for severe pain back then. Good luck these days. Our opioid policies are an outright abomination, something I've been writing about since 2013.
For short-term mild or moderate pain and inflammation, NSAIDs, such as Advil (ibuprofen) or Aleve (naproxen) are pretty good drugs. But some people can't take them at all, primarily because of gastrointestinal side effects. Long-term use can cause bleeding ulcers, even in people who don't suffer from heartburn-like symptoms.
Imagine a drug that worked as well as or maybe even better than Aleve, but without gastrointestinal toxicity. It would be a medical miracle. There isn't such a drug yet, but, thanks to some very clever chemistry and pharmacology, it's now a possibility. Wow.
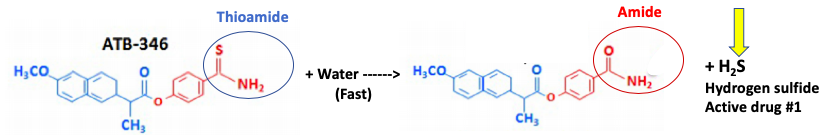
Antibe is trying to develop a pro-drug (2) of naproxen called ATB-346, which can eliminate GI side effects by having the drug bypass the stomach before releasing the naproxen. But ATB-346 takes it a step further. It was designed to also release small amounts of hydrogen sulfide, the poisonous gas from rotten eggs, upon decomposition. Best yet, the hydrogen sulfide isn't just coming along for the ride; it can also act as an antiinflammatory agent. Two drugs for the price of one. Amazing.
HOW DOES IT WORK? (3)
The hydrogen sulfide wasn't incorporated into ATB-346 by accident. Endogenous gaseous mediators (carbon monoxide and nitric oxide are the other two) are involved in multiple cellular processes, including cell repair, inflammation, and smooth muscle tone. (A 2009 review covers this topic.)
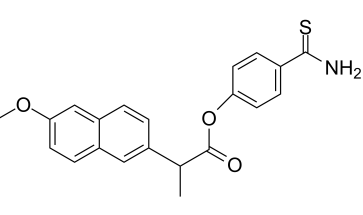
The chemical structure of ATB-346
So how do you make a molecule that contains two different anti-inflammatory drugs that is also safe? It's all about using chemistry and pharmacology together to solve two problems. Here's how it works:
Step 1 - Hydrolysis of the thioamide to an amide releases hydrogen sulfide (fast).
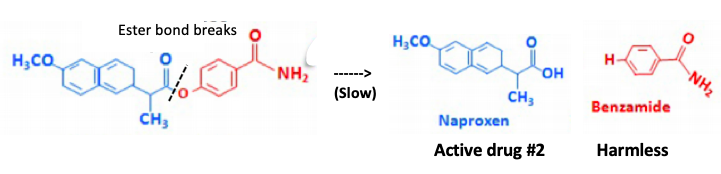
Step 2 - The ester bond breaks releasing naproxen (active drug) and benzamide, a harmless byproduct (slow).
IS IT SAFE?
In a Phase IIb (4), double-blind gastrointestinal safety trial, ATB-346 was far superior to naproxen.
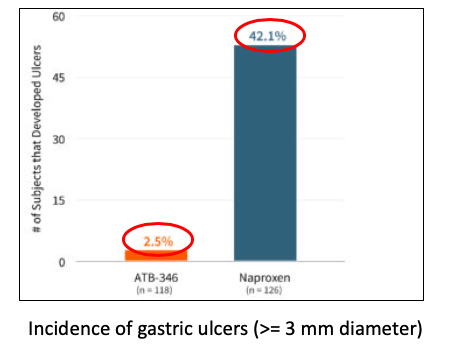
Source: Antibe Therapeutics
During a two-week treatment period, 53 out of 126 (42.1%) volunteers who got naproxen (500 mg, twice per day) developed ulcers while in the ATB-346 group (250 mg, once per day) 3 out of 118 (2.5%) people did. If you're wondering whether the difference in the daily doses (naproxen was four-times that of ATB-346) is the reason for the difference that is a valid point. However, this trial tells us nothing about the effective dose of ATB-346.
A Phase IIa efficacy trial at a dose of 250 mg trial provides this information.
HOW WELL DOES IT WORK?
Very well, it would seem.
A previous Phase IIa trial of 12 participants with osteoarthritis compared the efficacy of ATB-346 (single dose, 250 mg) with that of naproxen and also Celebrex in pain reduction on the WOMAC scale. People who were given ATB-346 had a reduction of about 4 units – roughly the same as naproxen and Celebrex – after 4 days and 7.6 units after 10 days.
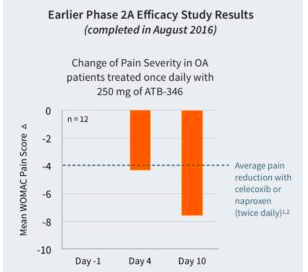
Source: Antibe Therapeutics
WHAT'S NEXT?
Antibe began a much larger Phase IIb, multi-site trial in early 2019 to establish the optimal dose for Phase III trials. The results should be out soon (Antibe says Q1 2020).
PREDICTION
The number of drugs that have died after Phase II trials is depressingly long. They primarily fail due to the lack of efficacy (safety has been established in Phase I, although safety surprises in Phase II aren't uncommon). There is little question that a 250 mg dose of ATB-346 is far safer than a standard dose of naproxen. The pain reduction data look impressive, but keep in mind that it is based only on 12 people.
You have to be out of your gourd to put money on a drug based on Phase II data, especially since we are talking about a novel drug in a novel series with no precedent.
Nonetheless, I'm going to go out on a limb. The Phase IIa/b data look impressive enough that I'm going to give this one a thumbs up.
Keep your arthritic fingers crossed that I'm right.
NOTES:
(1) ATB-346, if successful, will be used for the treatment of chronic, not acute, pain. The company has plans to develop another non-opioid drug for acute pain, but this is in a much earlier stage.
(2) A pro-drug is a chemically modified structure of a known drug that is inactive until the active species is liberated by metabolism. I have written in detail about Vyvanse, a pro-drug of Adderall, works. ATB-346 is technically not a pro-drug since it breaks down to two active species.
(3) I have provided only a quick look at how ATB-346 breaks down to form H2S and naproxen. The details of how and where this takes place are beyond the scope of this article, but I will follow up with an article on the mechanisms involved




