
Back in March, I gave an update on the progress of otenaproxesul (aka ATB-346), an experimental hydrogen sulfide-based NSAID that is being developed in Canada by Antibe Therapeutics. Although otenaproxesul is a derivative of naproxen, it isn't just another NSAID; data from Phase 2 clinical trials suggested that the drug was more potent than naproxen and virtually devoid of gastrointestinal toxicity that limits the utility of NSAIDs.

Source: Antibe website
If successful, otenaproxesul would become the only significant advance in pain control since the 19th century when aspirin and heroin were invented – a vitally important unmet medical need. Things looked good until they didn't.
Those of you who are familiar with drug discovery research are probably not the least bit surprised that otenaproxesul hit a snag earlier this week. Snags are the rule, not the exception in drug discovery, where failure is depressingly common.
Although the primary drawbacks of NSAIDs are GI and kidney toxicity, there are 78 organs in the human body, which gives experimental drugs an extra 76 organs to screw up. Apparently, this is just what happened. According to a press release from Antibe, three patients who had taken the high dose (more on this later) of otenaproxesul saw their liver enzymes rise and not by a little. Liver transaminase levels soared to five times the upper limit, which triggered a "required pause."
Forty-two subjects were enrolled in the trial; 35 completed a 28-day treatment period, while seven received the drug for 21 days. They received a daily dose of either 75 mg or 100 mg. The three enrollees who experienced spikes in their liver transaminase levels were all in the 28 day/100 mg group. No one saw this coming.
Although the treatment duration was longer than in previous Antibe studies, these observations were unexpected given the results of efficacy, safety and pharmacokinetic studies conducted to date.
Antibe press release, August 3, 2012
Wall Street didn't see this coming either.
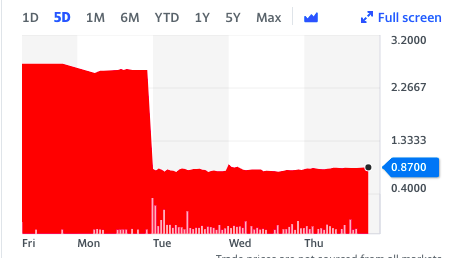
Antibe stock price July 30th - August 5th. Source: Yahoo Finance. (Guess what schmuck owns the stock.)
Unanswered questions
1. This trial was designed to evaluate drug safety, not efficacy. (The company calls it an "absorption, metabolism, and excretion (AME) study.") Given that safety was the endpoint, I'm a little surprised that the doses chosen were 75 mg and 100 mg – rather similar. More commonly, a safety trial would evaluate three different doses that are significantly different from each other, for example, 25, 50, and 100 mg. A larger range of doses would (presumably) make it possible to look for a dose-response relationship, which can help determine whether a given effect is real or artifactual.
2. Three of 35 enrollees who completed the 28-day dosing protocol had a spike in liver enzymes, but this spike was seen in only the high dose group. And this spike, which is anything but subtle, was not seen in earlier trials. This is not easily explained.
3. Despite the significant elevation in liver transaminases in three enrollees, there were no other markers that might suggest liver toxicity.
Best Guess
I don't know how Antibe makes this problem go away, especially since otenaproxesul would (presumably) be used chronically for pain and inflammation. Even if the drug is given at a lower dose, there will always be a red flag attached to it. And it is ironic (unfortunately so) that if otenaproxesul is withdrawn, it would be because of toxicity typical of Tylenol rather than that of traditional NSAIDs.
I hope I'm wrong.
Disclaimer: My IRA contains some a pathetically small amount of Antibe stock.



