In a pandemic, will physicians, who determine that the potential benefit of Ivermectin outweighs its well-documented risks for their patients, finally once again be given free rein to practice medicine?
Why can’t a drug approved and used in humans for 35 years, with excellent safety margins, and 3.7 billion doses administered worldwide, and with almost uniformly demonstrated benefit in all stages of COVID-19 infection, get a decent day in court?
The most recent updated meta-analysis of worldwide ivermectin use in COVID-19 disease shows risk reductions
- 85 % for prophylaxis (n=8,789)
- 81% early disease (n=1,937 subjects)
- 43%hospitalized patients (n=6,831 subjects)
- 76% overall reduction in mortality (n = 7,267 subjects)
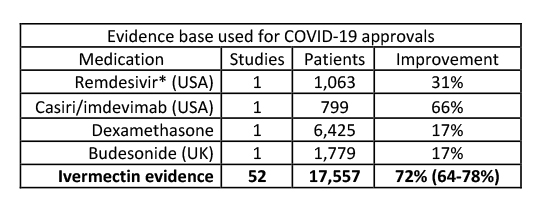
In aggregate, this data far outstrips the results felt sufficient to recommend Remdesivir, Casiri/imdevimab, dexamethasone, and budesonide to treat symptomatic COVID-19.
In January 2021, NIH upgraded its ivermectin stance from ‘not recommended’ to ‘neither recommended nor not recommended’ based upon a limited set of randomized controlled trials (RCT). If only considering RCTs, the ongoing meta-analysis found a 64% observed improvement in 27 studies involving 4,489 subjects.
Why the hesitancy to endorse? The NIH determined that several of the RCTs could be subject to bias, thus reducing overall confidence in a proven beneficial effect.
The latest Ivermectin Trial
A recent RCT, published in JAMA, is a perfect case of potential bias, ironically, in the opposite direction. This RCT in young mildly symptomatic COVID found a two-day non-statistically significantly shorter time to symptom resolution with ivermectin vs. placebo.
- Seventy-five consecutive subjects were dropped from the analyses when it was discovered they all received ivermectin rather than being randomized, raising questions about the rigor of study procedures.
- Placebo subjects had so few symptoms that the prespecified primary outcome had to be changed.
- At the time of the study, ivermectin was freely distributed to that city’s population with widespread acceptance and adoption. Subjects who took ivermectin for five days before the study were excluded, but ivermectin’s active metabolites have a 72-hour half-life, and significant biological effects can be seen up to one month after a single dose.
- Placebo subjects may have been “unblinded” to their grouping. During the initial study period, the taste of the placebo was clearly distinguishable, and no validation of taste equivalency is reported in the second period. Given the availability of information on social media and that multiple family members could be recruited from the same household during period 2, how hard would it be for a subject to conclude whether they received active drug
In other words, could the placebo group have been exposed to clinically relevant quantities of ivermectin? Maybe. Scheim noted that the side effects specific for high doses of ivermectin and not shared with COVID-19 symptomatology were identical between ivermectin and placebo groups. Unfortunately, no serum samples were collected to test ivermectin exposure.
What about other outcomes of interest?
The authors kindly shared their data with Wiseman and Kory, who analyzed for symptom persistence (SP) at day 21 (the converse to symptom resolution – the primary outcome) in the original dataset of 398 subjects combined with the excluded group of 75 known to have gotten ivermectin. In the original reported cohort of 398 subjects, ivermectin non-significantly reduced SP 18 vs. 21% in the placebo arm. When they compared the excluded ivermectin group of 75 to the remaining 198 subjects in the placebo groups, ivermectin reduced SP 9.3% vs. 21.2%. Hence several favorable signals were detected in a study with several structural biases against ivermectin.
A word about safety
There were no serious ivermectin-related adverse events. Reports of dizziness (40.0%), blurred vision (10.7%), and nausea (24%) were high but resolved within a median of 2 days or less, all consistent with the drug’s label. A cumulative incidence of one serious adverse event per million was reported over the first 11 years of use during mass global administration.
Summary
As published, the study by Lopez-Medina and colleagues is flawed, biased against ivermectin. It fails to meaningfully contradict accumulating data in favor of the efficacy and safety of ivermectin in COVID-19 disease. Significantly, according to the analysis of Wiseman and Kory, the study supports its use.
Troubling questions
The NIH (which has no statutory authority overprescribing practices) has determined that patients should only be permitted access to ivermectin if they enroll in a clinical trial. Yet unless I live in or near one of four small US trial sites, there are no US clinical trials into which I may enroll. Recently the NIH has rolled out the ACTIV-3b study, an adaptive protocol to test promising agents on the ‘standard of care’ background of Remdesivir and dexamethasone. Will ivermectin be allowed to compete against patented investigational drugs with little to no supportive human data? What about prophylaxis? To the author’s knowledge, only vaccines for prevention and costly monoclonal antibodies for outpatients are receiving government funding.
Regardless of how definitive trials of ivermectin for COVID-19 turn out, we owe our respect and gratitude to those who have put in the long hours to conceive of and then test new treatments during this pandemic. There should be no place for politics, ad hominem attacks, censorship, or cancel culture in the scientific debate.
Note: the views expressed here are strictly those of the author and not of any other entity.
