In a new article in Nature, author Max Kozlov asks the question, "COVID drug Paxlovid was hailed as a game-changer. What happened?" It's an excellent question.
When clinical trial data for the antiviral drug Paxlovid emerged in late 2021, physicians hailed its astonishing efficacy — a reduction of nearly 90% in the risk of severe COVID-19. But more than a year later, COVID-19 remains a leading cause of death in many countries, and not only in low-income nations where the drug is in short supply. In the United States, for example, hundreds of people still die from COVID-19 each day.
M. Kozlov, Nature 613, 224-225 (2023) doi: https://doi.org/10.1038/d41586-022-04576-6
Let's try to understand why one of the two remaining treatments (1) for COVID is being underused. It's partly due to bad press, but, for the most part, articles about the rebound do not blame the drug, even though the name "Paxlovid rebound" implies this. Of course, there are exceptions:

Uber-quack Joe Mercola gets it wrong. As usual.
The real problem is that the name itself is technically incorrect and misleading; a Google search for "Paxlovid rebound" turned up 92,000 hits. Ideally, that number should be zero because the term implies that Paxlovid itself is somehow responsible for the infection reoccurring once the drug – a five-day course – is stopped. This is dead wrong, just like Mercola.
There is now ample evidence that Paxlovid has nothing to do with this rebound; it is the virus that causes it, something my colleague Dr. Henry Miller and I examined in a post on the Genetic Literacy Project site in 2022. Let's look at the evidence.
It's not just Paxlovid
A 2022 medRxiv article (non-peer-reviewed preprint, retrospective study) compared the rate of disease rebound in people who took Paxlovid and Lagevrio (generic name molnupiravir) – the only other FDA-approved antiviral Covid drug (2).
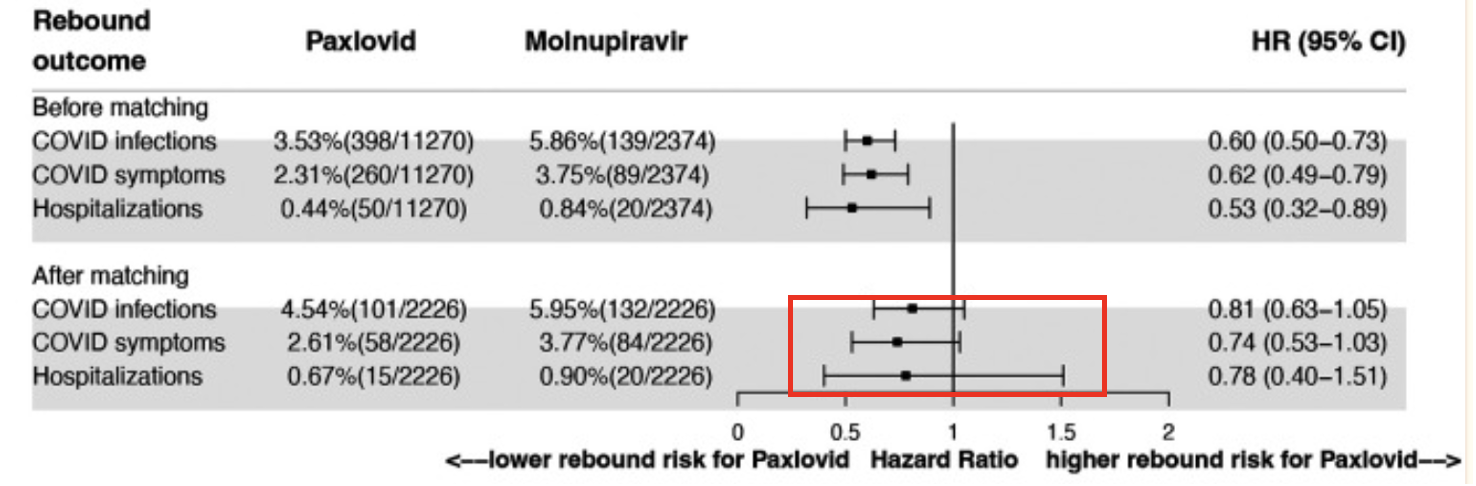
Above is a forest plot of rebound percentages (follow-up period seven days) of patients who took either Paxlovid or Lagevrio (in a forest plot, any horizontal line that crosses the HR = 1 vertical line means that there is no statistical significance). The plot at 30 days (not shown) is essentially identical. Note that in the top three lines of the table, there appears to be a significant difference in disease rebound in patients who took Paxlovid compared to those who took Lagevrio. But the effect is not real; it can be explained by the difference in the underlying health of patients who took each drug. Those who took Lagevrio were older and had more co-morbidities (3). Once corrections were made to account for the underlying health og patients in each group, the difference disappeared (red box).
It would be just as (in)correct to refer to "Paxlovid rebound" as "Lagevrio rebound."
In summary, COVID-19 rebound (infection, symptoms, and hospitalizations) occurred for both drug treatments. The risks for rebound did not differ between Paxlovid and Molnupiravir, indicating that COVID-19 rebound is not unique to Paxlovid.
Wang, et. al., Version 1. medRxiv. Preprint. 2022 Jun 22. doi: 10.1101/2022.06.21.22276724
Rebound in Paxlovid-treated vs. untreated patients
A September 2022 letter in NEJM extracted data from Pfizer's EPIC-HR (Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients)) Phase III trial (4) of about 2,200 "symptomatic, unvaccinated outpatient adults at high risk for progression to severe coronavirus disease." Half of the group was treated with Paxlovid; the other half was not. According to the authors:
"From baseline through day 14, viral load rebound occurred in 23 of 990 patients (2.3%) in the nirmatrelvir–ritonavir group and in 17 of 980 (1.7%) in the placebo group."
In other words, there was not a statistically significant difference in the rate of rebound in the Paxlovid-treated and control groups.
Source: N Engl J Med 2022; 387:1047-1049 DOI: 10.1056/NEJMc2205944. Data included in Table S2
Another theory: Mutation
Others have suggested that the rebound might be caused by a mutation of the virus within a patient taking Paxlovid, forming a variant that was resistant to Paxlovid. Anyone with a knowledge of virology will probably dismiss this theory as far-fetched. Based on research on HIV and hepatitis C – the same playbook used to develop Paxlovid – the chance of a five-day course of a protease inhibitor generating a new Paxlovid-resistant Covid strain, which would then reinfect the same patient is remote. Furthermore, all of the clinically significant variants and subvariants of Covid have differed only by mutations in the spike protein, not its main protease (Mpro) enzyme.
Additionally, there are scattered reports of sequencing the genome of "before and after" infection. These have shown that the virus is unchanged, not mutated. And David Ho, famous for his award-winning research on AIDS, also doubts that Paxlovid resistance plays a part in Covid rebound.
Of course, completely ruling out the resistance theory will require sequencing the before and after genomes of a large number of people who have suffered a rebound – something I don't see happening anytime soon.
Bottom Line
Although there is no definitive evidence that Paxlovid does not cause Covid rebound, there is, when taken as a whole, a convincing argument that this is true. And there is no evidence at all that it does. Based on clinical data, knowledge of the mechanism of viral mutation, and common sense, my best guess is that either an inadequate treatment period, differences in individual metabolism of the drug, or both support my contention that the Paxlovid rebound is really a Covid rebound. Blame the virus, not the medicine. (5)
NOTES:
(1) The arsenal against Covid continues to shrink. The monoclonal antibodies that used to be effective in preventing serious disease no longer work against newer subvariants, leaving us with nothing except the two oral (and one IV) drugs. This needs to change.
(2) Molnupiravir operates by an entirely different mechanism and is less effective than Paxlovid.
(3) Because of drug-drug interactions, Paxlovid cannot be in combination with some essential heart drugs, antiarrhythmics, and blood thinners. This explains why people who must take molnupiravir instead of Paxlovid have poorer health and more risk factors."
(4) The EPIC-HR trial demonstrated that Paxlovid was 89% effective in preventing Covid progression to severe disease compared with placebo.
(5) If you want to blame the drug for anything I (and many others who I know) got a nasty (but tolerable) case of "Paxlovid mouth," something that inspired me to write a rather terrible blues song about it.




