
It's a Friday summer weekend. Brain is fried. Concentration, which isn't all that swift on a good day, is... what's that word?
Which means that it's a good time to do something fun.
Here is a "5-minute crafts" video clip of yellow and blue water, which appears to demonstrate that when you place one color on top, you get three layers of colors, whereas the other results in just one.
For extra fun, I've written an explanation for why all this stuff is happening. I accept Krugerrands.
Experiment # 1: Yellow and blue make green. But slowly.

Explanation: Warm water is less dense than cold. So the warm layer floats on top. Once the warm water cools, the two will mix completely. You can see this beginning at the green interface.
Experiment # 2: Yellow and blue make green. But fast.
This time, yellow dye is added to the warm water. The excitement grows...

Explanation: Experiment #2 is the opposite of #1. This time, the cold (blue) water is denser, so it sinks immediately when added to the yellow solution. The two colors to mix immediately.
Experiment # 3: A sucking candle.
Explanation: Just like with water, hot air is less dense than cold. But this experiment is a little different. Unlike liquids, which expand imperceptibly, gases expand like crazy when heated. Once the glass is placed over the candle, the air is heated and it expands. The resulting pressure forces some air out of the glass (the bubbles). When the oxygen is consumed, the flame goes out. Then the air cools and contracts. This results in a vacuum which draws the blue solution into the glass.
Experiment #4: Dancing Grapes.
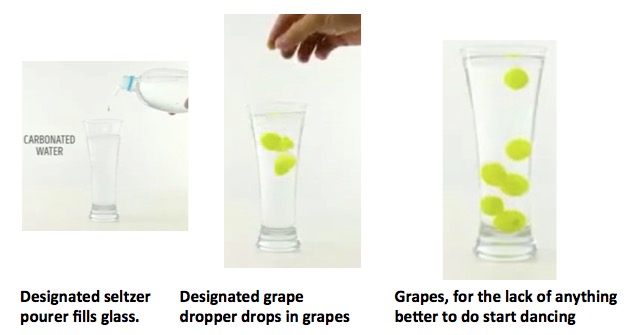
Grapes are denser than water (and also carbonated water), so when they are dropped into the glass, they sink to the bottom. Carbon dioxide bubbles adhere to the surface of the grapes. When enough of the bubbles have latched onto the grape, it becomes buoyant and floats to the top. The CO2 bubbles burst and the grape sinks again where it picks up some more CO2 (sung to the tune of "The Itsy Bitsy Spider").
Experiment #5: A five-layer cake without the cake.

Explanation: Figure it out for yourself. This should be good. Feel free to leave answers in the comments section.
Have a peaceful, chemical-free weekend.



